Arene Chemistry - Miller, Jonathan
advertisement
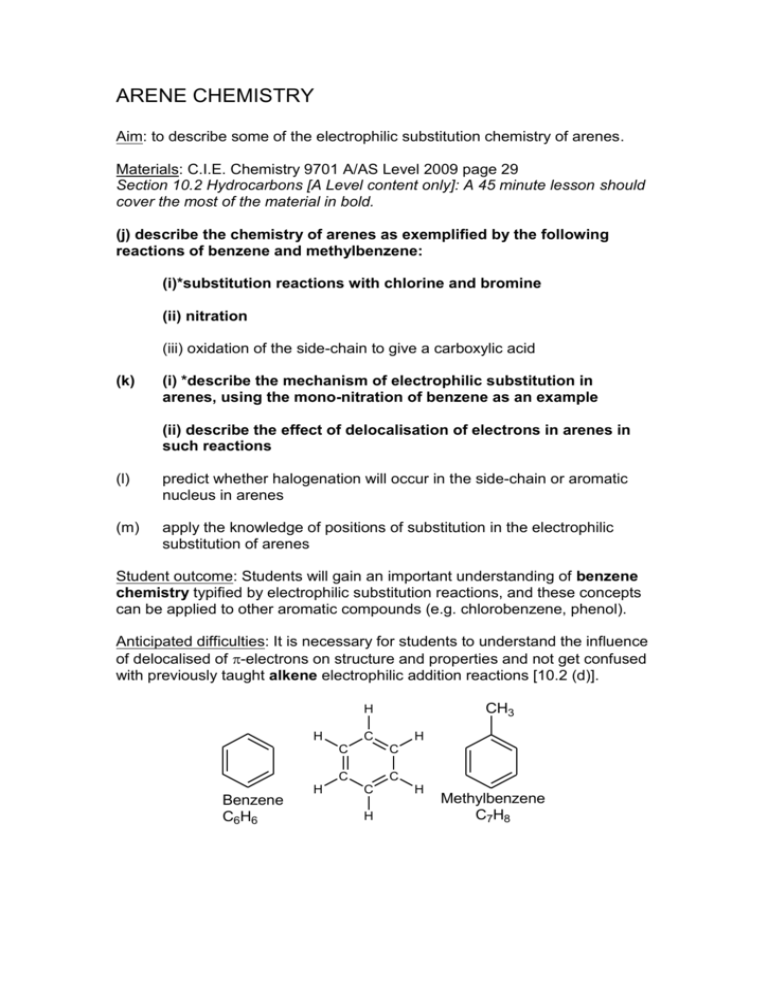
ARENE CHEMISTRY Aim: to describe some of the electrophilic substitution chemistry of arenes. Materials: C.I.E. Chemistry 9701 A/AS Level 2009 page 29 Section 10.2 Hydrocarbons [A Level content only]: A 45 minute lesson should cover the most of the material in bold. (j) describe the chemistry of arenes as exemplified by the following reactions of benzene and methylbenzene: (i)*substitution reactions with chlorine and bromine (ii) nitration (iii) oxidation of the side-chain to give a carboxylic acid (k) (i) *describe the mechanism of electrophilic substitution in arenes, using the mono-nitration of benzene as an example (ii) describe the effect of delocalisation of electrons in arenes in such reactions (l) predict whether halogenation will occur in the side-chain or aromatic nucleus in arenes (m) apply the knowledge of positions of substitution in the electrophilic substitution of arenes Student outcome: Students will gain an important understanding of benzene chemistry typified by electrophilic substitution reactions, and these concepts can be applied to other aromatic compounds (e.g. chlorobenzene, phenol). Anticipated difficulties: It is necessary for students to understand the influence of delocalised of -electrons on structure and properties and not get confused with previously taught alkene electrophilic addition reactions [10.2 (d)]. CH3 H H C C C Benzene C6H6 H H C C C H H Methylbenzene C7H8 Electrophilic Substitution Reactions of Arenes Benzene takes part in a variety of substitution reactions with electrophilic reagents (positively charged). If chlorine is passed through benzene at room temperature in the presence of a suitable catalyst (the Lewis acid aluminium trichloride), substitution takes place. However, due to delocalisation, no electrophilic addition occurs (compare with alkenes). Cl AlCl3 + Cl2 + HCl chlorobenzene Similarly, bromine in the presence of the aluminium tribromide [or Iron(III) bromide]: C6H6 + Br2 AlBr3 C6H5Br + HBr When treated with a mixture of concentrated nitric acid and concentrated sulphuric acid at room temperature, nitrobenzene is formed: NO2 H2SO4 + HNO3 + H2O nitrobenzene Mechanism of Electrophilic Substitution: Nitration of Benzene: The reaction occurs in several stages. 1. The Nitronium ion (NO2+) is generated: H H2SO4 + H O NO2 HSO4- + + O H H + O H + NO2 H2O + O N O nitronium ion (electrophile) NO2 2. The electrophile reacts with benzene by addition: H NO2 H H + NO2 + NO2+ NO2 + The positive charge is delocalised over three of the carbon atoms; the ion is a resonance hybrid (mixture) of three structures above and this delocalisation stabilizes the developing positive charge making benzenes and arenes more reactive than alkanes. Remember the more spread out the positive charge is around the ring and lower in energy! (The curly arrows represent a movement of electrons). H NO2 positive charge spreads around the ring + 3. In the final step, it is deprotonated to give nitrobenzene: H NO2 NO2 + + HSO4- + H2SO4 In a similar way, we can show its reaction with chlorine. A (+ -) dipole is created as the halogen approaches the arene because the electron clouds repel each other. + Cl Cl Cl + - AlCl3 + AlCl4- H Concept check: Ask the students (to see if they understand): Why does benzene not undergo electrophilic addition reactions? The three double bonds are delocalised and are more stable than an alkene. It reacts in a different manner undergoing electrophilic substitution, for example with chlorine in the presence of aluminium trichloride catalyst. Remember in delocalised systems the electrons move around the ring. Methylbenzene is an arene. Do you think it will undergo electrophilic substitution reactions? Why Yes. The ring is delocalised in the same way as benzene. We will look at the special chemistry of methylbenzene in the next lesson. What type of reaction is this? Why is aluminium bromide used? C6H6 + CH3CH2 Br AlBr3 C6H5 C2H5 + HBr ethylbenzene Benzene reacts with an alkyl halide in the presence of aluminium tribromide catalyst to give an alkylbenzene (ethylbenzene). It is another example of an electrophilic substitution reaction. Evidence of concept understanding beyond A-Level Pyridine, C5H5N, a colourless liquid, has aromatic properties and undergo some electrophilic substitution reactions. Other similar heterocyclic compounds behave in the same way. However, the nitrogen atom is basic and readily accepts a proton to form an ionic compound. Draw a diagram to show how the nitrogen atom assists in producing an aromatic ring. N: N .. N .. Aromatic nature of pyridine Nitrogen of group V has two SP2 hybrid orbitals, one P orbital and one lone pair of electrons. One electron from the P orbital is used in delocalised bonding in the six-membered ring. In the diagram above the electron cloud therefore becomes delocalised. It is the lone pair of electrons that make pyridine basic in nature, but do not take part in the delocalised ring!