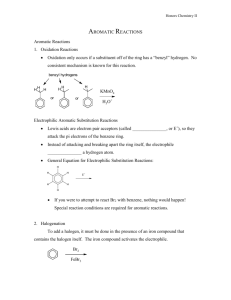
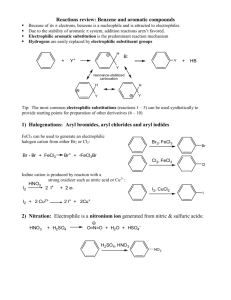
EAS Reactions Summary: Electrophilic Aromatic Substitution
advertisement
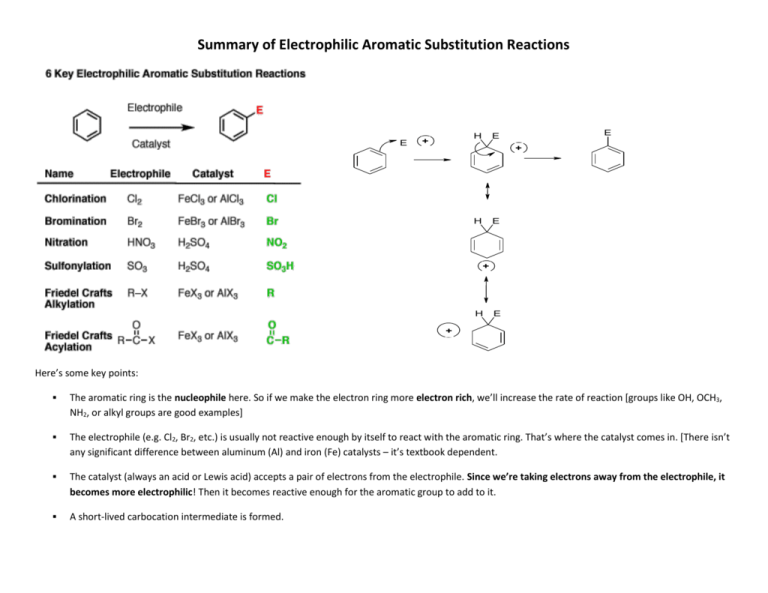
Summary of Electrophilic Aromatic Substitution Reactions E H + E E + H E + H E + Here’s some key points: The aromatic ring is the nucleophile here. So if we make the electron ring more electron rich, we’ll increase the rate of reaction [groups like OH, OCH3, NH2, or alkyl groups are good examples] The electrophile (e.g. Cl2, Br2, etc.) is usually not reactive enough by itself to react with the aromatic ring. That’s where the catalyst comes in. [There isn’t any significant difference between aluminum (Al) and iron (Fe) catalysts – it’s textbook dependent. The catalyst (always an acid or Lewis acid) accepts a pair of electrons from the electrophile. Since we’re taking electrons away from the electrophile, it becomes more electrophilic! Then it becomes reactive enough for the aromatic group to add to it. A short-lived carbocation intermediate is formed. Finally, a weak base comes along and re-forms the double bond, restoring aromaticity. This step is a lot like the last step of the E1 elimination mechanism. Watch the following video links and observe the common principles associated with the following EAS reactions: 1) bromination of benzene (or chlorination of benzene) 2) Friedel-Crafts Alkylation 3) Friedel-Crafts Acylation 4) Nitration of benzene 5) Sulfonation of benzene After watching the following videos, access the following link that contains a summary of these reactions & use that summary table to complete the table below: Summary of EAS reactions Substrate (& name) Reagent Electrophile Cl2 / Fe or FeCl3 Cl+ Electrophilic substitution intermediate Cl H + halogenation H Br + Product H NO2 + HNO3 /H2SO4 SO3H+ CH3CH2+ H + H + and AlCl3 + H + Isopropyl chloride & FeCl3 + O O + O + H Now that you’ve completed this EAS summary sheet, choose one of the following reactions and draw the complete mechanism for that reaction: Formation of chlorobenzene Formation of methylbenzene Formation of nitrobenzene What is a weak base + why is a weak base needed for most of these reactions?