lect5
advertisement

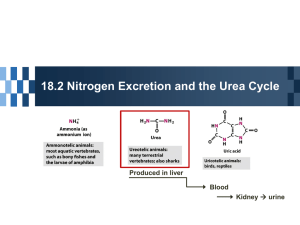
BIOC/DENT/PHCY 230 LECTURE 5 glu UREA synthesised mainly in liver maintains N in a soluble, non-toxic form transported in blood to kidney for excretion allows for the elimination of 2 nitrogens O H2N C NH2 How does nitrogen enter the urea cycle? O H2N C O P carbamoyl phosphate aspartate Carbamoyl phosphate synthesised from carbon dioxide and ammonia ammonia comes from deamination of glutamine and glutamate energy requiring reaction catalysed by carbamoyl phosphate synthetase (CPS) CO2 + NH3 + 2ATP carbamoyl- P + 2ADP + Pi Aspartate aspartate is generated by transamination glutamate + oxaloacetate a-KG + aspartate Entry of substrates into the urea cycle CAC Urea cycle 1= CPS 2= ornithine transcarbamoylase 5= arginase Recycling fumarate in the CAC CAC Overall equation for urea cycle CO2 + NH3 + 3ATP + 2H2O urea + 2ADP + 2Pi +AMP + PPi + fumarate urea synthesis is an energy demanding process the equivalent of 4 ATP are consumed for each molecule of urea synthesised Regulation of urea cycle Two major points of regulation: concentration of urea cycle enzymes CPS activity CPS activity CPS is allosterically regulated N-acetylglutamate activates CPS N-acetylglutamate synthase is activated by arginine positive feedback Disorders of urea cycle enzymes: 1=CPS 2= ornithine transcarbamoylase Nitrogen compounds in urine Urea - major nitrogen excretion product. NH4+ - produced in the kidney by deamination of glutamine. Reduces body acidity because the process removes protons. Uric Acid - the final metabolic product of purinenucleotide degradation. Creatinine - derived in skeletal muscles, by spontaneous cyclisation of creatine & phosphocreatine. Fates of amino acid carbon skeletons Carbon skeletons can be glucogenic or ketogenic Biosynthesis of amino acids The take home message urea is a small organic metabolite used to eliminate excess nitrogen from the body the urea cycle requires a substantial input of energy CPS is the key regulatory step of the urea cycle other N compounds are excreted in the urine carbon skeletons can be recycled or oxidised availability of carbon skeletons determines dietary requirements for amino acids