Carbamoyl phosphate synthetase I
advertisement
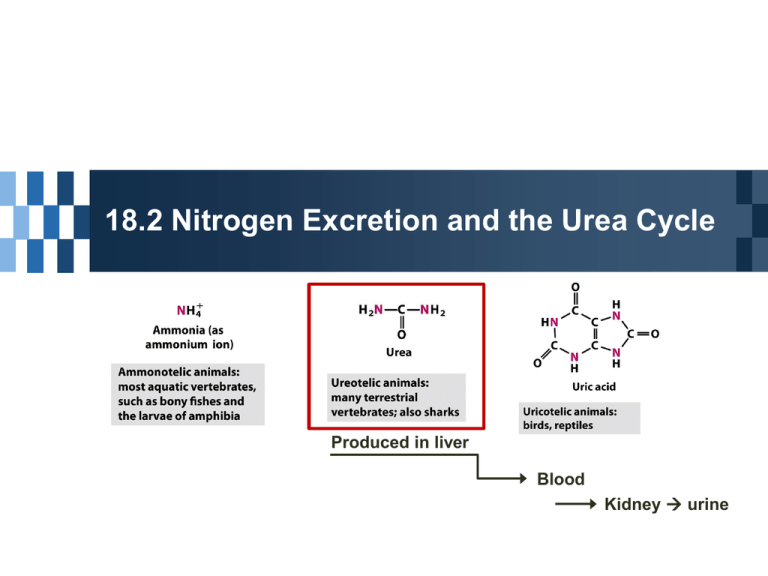
18.2 Nitrogen Excretion and the Urea Cycle Produced in liver Blood Kidney urine Urea Cycle in Mitochondria Formation of carbamoyl phosphate; preparatory step NH4+ + HCO3- + 2 ATP carbamoyl phosphate + 2 ADP + Pi Carbamoyl phosphate synthetase I - ATP-dependent reaction 1st step in the urea cycle; Ornitine + carbamoyl phosphate citrulline + Pi Ornitine transcarbamoylase Urea Cycle in Cytosol 2nd step; formation of argininosuccinate Incorporation of the second N from aspartate Argininosuccinate synthetase ATP requirement Citrullyl-AMP intermediate 3rd step; formation of arginine & fumarate Arginosuccinase; only reversible step in the cycle 4th step; Cleavage of arginine to urea & ornithine Arginase Asparatate-argininosuccinate shunt Metabolic links between citric acid and urea cycles In cytosol Fumarate to malate citric acid cycle in mitochondria In mitochondria OAA + Glu a-ketoglutarate + Asp urea cycle in cytosol Energetic cost • Consumption 3 ATP for urea cycle • Generation Malate to OAA 1 NADH = 2.5 ATP Regulation of the Urea Cycle Long term regulation Regulation in gene expression Starving animals & very-high protein diet Increase in synthesis of enzymes in urea cycle Short term regulation Allosteric regulation of a key enzyme Carbamoyl phosphate synthetase I Activation by N-acetylglutamate Treatment of genetic defects in the urea cycle Genetic defect in the urea cycle ammonia accumulation; hyperammonemia Limiting protein-rich diet is not an option Administration of aromatic acids; benzoate or phenylbutyrate Administration of carbamoyl glutamate Supplement of arginine 18.3 Pathways of Amino Acid Degradation Amino Acid Catabolism Carbon skeleton of 20 amino acids Conversion to 6 major products - pyruvate - acetyl-CoA - a-ketoglutarate - succinyl-CoA - fumarate - oxaloacetate Glucogenic or Ketogenic Amino Acids Ketogenic amino acids Conversion to acetyl-CoA or acetoacetyl-CoA ketone bodies in liver Phe, Tyr, Ile, Leu, Trp, Thr, Lys Leu : common in protein Contribution to ketosis under starvation conditions Glucogenic amino acids Conversion to pyruvate, a-ketoglutarate, succinyl-CoA, fumarate, and OAA glucose/glycogen synthesis Both ketogenic and glucogenic Phe, Tyr, Ile, Trp, Thr Enzyme cofactors in amino acid catabolism One-carbon transfer reactions ; common reaction type, involvement of one of 3 cofactors Biotin ; one-carbon tranfer of most oxidized state, CO2 Tetrahydrofolate (H4 folate) ; One-carbon transfer of intermediate oxidation states or methyl groups S-adenosylmethionine ; one-carbon transfer of most reduced state, -CH3 Tetrahydrofolate folate (vitamin) to H4 folate Dihydrofolate reductase Primary source of one-carbon unit Carbon removed in the conversion of Ser to Gly Oxidation states of H4 folate ; One-carbon groups bonded to N-5 or N-10 or both - Methyl group (most reduced) - Methylene group - Methenyl, formyl, formimino group (most oxidized) Interconvertible & donors of onecarbon units (except N5-methyltetrahydrofolate) S-adenosylmethionine (adoMet) Cofactor for methyl group transfer Synthesized from Met and ATP Methionine adenosyl transferase Unusual displacement of triphosphate from ATP Potent alkylating agent Destabilizing sulfonium ion inducing nucleophilic attack on methyl group Six amino acids are degraded to pyruvate Ala, Trp, Cys, Ser, Gly, Thr pyruvate acetyl-CoA citric acid cycle or gluconeogenesis Interplay of PLP and H4folate in Ser/Gly metabolism 3rd pathway of glycine degradation - D-amino acid oxidase detoxification of D-amino acid high level in kidney - Oxalate crystals of calcium oxalate (kidney stones) Seven Amino Acids Are Degraded to Acetyl-CoA Trp, Lys, Phe, Tyr, Leu, Ileu, Thr acetoacetyl-CoA acetyl-CoA Intermediates of Trp catabolism be precusors for other biomolecules Catabolic pathways for Phe & Tyr Phe & Tyr are precusors dopamine norephinephrine, epinephrine melanin











