Sample lab report exp1
advertisement

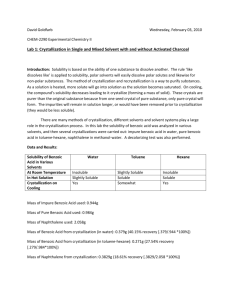
Sample lab report These notes are to supplement, not supplant the other notes I gave you on these sections. 8. Procedure: Give yourself enough info so that you can do the experiment. Include amounts and times so that you can do the experiment by these notes alone. You will have the lab handout to refresh yourself on how to work some of these new procedures, if you really need it. 9. Observations: These will parallel the procedure section. At a minimum, record all color changes, phase changes, measurements (mass, etc). The more you record here, the easier it will be to recall what you did during the lab. This can be especially important if you need to explain a mistake you made. 10. Analysis/ 11. Discussion of results: These can easily combined into one section of most lab reports. This is where you restate your results and compare them to any literature values or other expectations. In the first lab, you should have a table of results for your mixture mp data. The table should include actual data as well as the average numbers you used for graph. A sample is below. The sample is incomplete and meant to give you an idea on how to do yours, but is not meant for you to cut and paste. None of the actual numbers or spellings of molecules cited here should be taken as authentic. You do NOT have to do your data in exactly this format as long as you have your data grouped in some way that makes sense, has a title, and gives the units. I encourage you to come up with your own format, for that is what you have to do for the rest of the year. You should also have at least one other table showing your mp, bp, and refractive index data as well as the literature values for these results. In addition, on a separate sheet of graph paper should be your eutectic graph, taking the whole page. Tables and graphs should have titles and numbers. Table 1. Class Melting Point Data of Cinamic Acid (C.A.)/Urea Mixtures (°C) 100% C.A./0% 131-133 80% C.A./20% 123-129 50% C.A./50% Urea Urea (result 2) Urea (result 2) 90% C.A./10% 127-130 … … Urea (result 1) 90% C.A./10% 123-128 … … Urea (result 2) 80% C.A./20% 121-125 … 0% C.A./100% Urea (result 1) Urea The literature m.p. (source: Merck Index) for pure Urea is 141°C while that of pure Cinamic acid is 166°C Table 2. Data of Melting Points(°C), Boiling Points(°C), and Refractive Index for Unknowns Unknown solid 131-133 Mixture of unknown G 127-130 Unknown Liquid 666 123G (mp) and benzoic acid (mp) (bp) 129 Pure Benzoic 127-130 Unknown Liquid 666 1.4567 Literature bp … Acid (mp) Raw Refractive Index Ethanol (Merck Index) Literature mp 128 Unknown Liquid 666 Temp 1.4587 Literature Refractive … Benzoic Acid corrected Refractive Index Index (Merck Index) Ethanol (Merck Index) Your eutectic curve should have a title, labeled horizontal axis and labeled on the vertical on both sides. If you use a graphing program, DO NOT try to draw any curves to fit the lines. Draw the points, then try drawing a smooth fit curve to fit those points. Including in your data analysis section would be any notes on how the curve was drawn. For instance, the graph was done using the upper value of the melting point range. When more than one range was reported, the average value was plotted. After presenting the data, you do some analysis. For example, you might say something along the lines of a smooth fit curve of the mixture melting points fit the data fairly well except for the data point for the 70% cinamic acid/30% urea was much too high. Ignoring that, the curve seems to indicate the eutectic point is somewhere between 50/50 and 40% cinamic acid/60% urea at approximately 40-50 °C. Unknown solid G seems to be benzoic acid. The mp of unknown G is close to the literature value for benzoic acid and the mp of the pure benzoic acid. Although the range of the mp taken in lab is a little large for pure substances, a mixture of Unknown G and pure benzoic acid has the same range without a drop in the number, indicating that Unknown G is benzoic acid and that the technique of mp needs to be refined by the experimenter. Unknown liquid 666 had a bp of … and a refractive index of …, which were both close to the literature values for ethanol. Therefore, unknown liquid 666 is ethanol. 12. Conclusion: This is where we summarize our data and say what we learned about the purpose. Brevity is good as long as the important stuff is covered. For instance… Unknown solid G is benzoic acid and unknown liquid 666 is ethanol. The class data for mixture mp yielded a smooth eutectic curve except that the data for 70% cinamic acid/30% urea seemed too high. This experimenter needs more practice with the mp technique to learn how to take tighter ranges for pure substances. Questions 1. … 2. …. 3. Etc.