Urea
advertisement

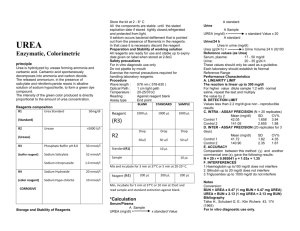
urea urea, also called Carbamide, the diamide of carbonic acid. Its formula is H2NCONH2. Urea has important uses as a fertilizer and feed supplement, as well as a starting material for the manufacture of plastics and drugs. It is a colorless, crystalline substance that melts at 132.7° C (271° F) and decomposes before boiling. Urea is the chief nitrogenous end product of the metabolic breakdown of proteins in all mammals and some fishes. The material occurs not only in the urine of all mammals but also in their blood, bile, milk, and perspiration. In the course of the breakdown of proteins, amino groups (NH2) are removed from the amino acids that partly comprise proteins. These amino groups are converted to ammonia (NH3), which is toxic to the body and thus must be converted to urea by the liver. The urea then passes to the kidneys and is eventually excreted in the urine. Urea was first isolated from urine in 1773 by the French chemist Hilaire Marin Rouelle. Its preparation by the German chemist Friedrich Wöhler from ammonium cyanate in 1828 was the first generally accepted laboratory synthesis of a naturally occurring organic compound from inorganic materials. Urea is now prepared commercially in vast amounts from liquid ammonia and liquid carbon dioxide. These two materials are combined under high pressures and elevated temperatures to form ammonium carbonates, which then decomposes at much lower pressures to yield urea and water. Because its nitrogen content is high and is readily converted to ammonia in the soil, urea is one of the most concentrated nitrogenous fertilizers. An inexpensive compound, it is incorporated in mixed fertilizers as well as being applied alone to the soil or sprayed on foliage. With formaldehyde it gives methylene urea fertilizers, which release nitrogen slowly, continuously, and uniformly, a full year’s supply being applied at one time. Although urea nitrogen is in none protein form, it can be utilized by ruminant animals (cattle, sheep), and a significant part of these animals’ protein requirements can be met in this way. The use of urea to make urea–formaldehyde resin is second in importance only to its use as a fertilizer. Large amounts of urea are also used for the synthesis of barbiturates. Urea reacts with alcohols to form urethanes and with malonic esters to give barbituric acids. With certain straight-chain aliphatic hydrocarbons and their derivatives, urea forms crystalline inclusion compounds, which are useful for purifying the included substances. Address : 5th Floor, No 203, Asadabadi St. Tehran, Iran. Postal Code : 1433913316 Tell : (+98 21) 88104101 – 5 Fax : (+98 21) 88104104 Email : Info@naturalbitumen.org www.NaturalBitumen.org , www.NaturalAsphalt.ir