Multivalent Metals and Ionic Compounds - Bennatti
advertisement

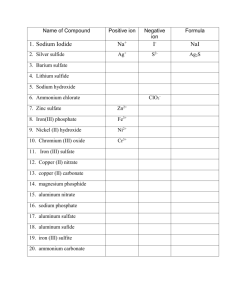
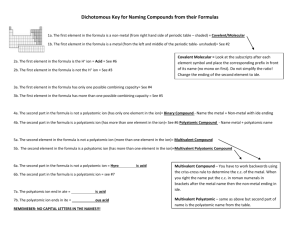
Multivalent Metals and Ionic Compounds Name____________________ Date______ The transition elements are generally multivalent. This means they can form more than one kind of ion. Iron is an example of a multivalent metal. It can form an ion with a +2 charge (Fe2+) or an ion with a +3 charge (Fe3+). When naming compounds containing a multivalent metal, Roman numerals are used to identify the charge. For example, nickel (III) has a charge of +3. When nickel (III) phosphide forms, the nickel atom loses 3 electrons and the phosphorus atom gains 3 electrons. Only one of each ion is needed as the charges add up to zero. The formula is NiP. Notice that when writing the formula, the Roman numeral is not included. It is included in the name of the compound. Complete the following table. The first one is done for you. Metal Nonmetal Iron (III) Iron (II) Manganese (II) Manganese (II) Lead (II) Lead (II) Copper (I) Copper (II) Chromium (II) Chromium (III) Chromium (II) Chromium (III) Chromium (II) Chromium (III) oxygen oxygen chlorine sulfur oxygen iodine oxygen chlorine phosphorus phosphorus fluorine fluorine sulfur sulfur Cation Charge Fe3+ Anion Formula of the Charge Compound O2Fe2O3 Name of the Compound Iron (III) oxide Some ions are made of more than one atom bonded together. They are called polyatomic ions. The formula for a polyatomic ion looks much like the formula for a compound with one important difference. While compounds are always neutral, polyatomic ions have a charge. For example: CO2 is the compound carbon dioxide CO32- is the polyatomic ion carbonate. It is made of 4 atoms bonded together with 2 more electrons in total than protons. Oppositely charged ions are attracted to each other. The metal calcium and polyatomic ion carbonate form the ionic compound calcium carbonate. Do not change the ending of the polyatomic ion when naming a compound containing a polyatomic ion. Ca2+ + CO32- calcium carbonate If a compound contains more than one polyatomic ion, parentheses are used. The formula for the polyatomic ion goes inside the parentheses. The number of the polyatomic ions needed goes outside the parenthesis. Ca2+ + NO2- Ca(NO2)2 NO2 is the nitrite ion. Two nitrite ions are needed to balance the charge of the calcium ion. The name of the compound is calcium nitrite. Al3+ + SO42- Al2(SO4)3 Three sulfate ions balance two aluminum ions. The name of the ion is aluminum sulfate. Use the table of ion to find the charge and name of the polyatomic ions. You do not need to memorize this table! Fill in the following table. The first one is done for you. Cation Al3+ Al3+ Na+ Li+ Mg2+ Mg2+ Li Ca2+ Na Anion name and formula CO32NO3CO32OHOHPO43PO43NO3NO3- Chemical Formula Al2(CO3)3 Name of the Compound Aluminum carbonate