CH 4 Aldehydes and Ketones Lecture Notes
advertisement

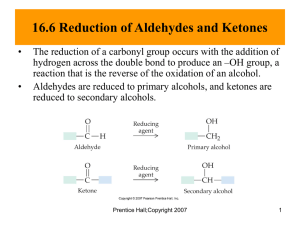
CH 4: Aldehydes and Ketones I. Introduction to the carbonyl group a. Polarity b. Bond angle - 1200 II. Compounds with a carbonyl group – functional groups a. Aldehyde - CHO b. Ketone: c. Carboxylic acid: RCOOH d. Esters e. Amides III. Aldehydes and Ketones a. Structure b. ?cyclic possible? i. For aldehydes - no ii. For ketones – yes IV. Naming Aldehydes – page 125 (do practice exercise) a. Locate longest C chain containing the aldehyde group b. Change ending of name from “e” to al i. Propanal – 3 C aldhehyde ii. Benzaldehyde c. Number the chain, CHO is C #1 d. Locate substituents, list in alphabetical order e. Comments – aldehyde group takes naming priority over alcohol group i. Alcohol as a substituent is “hydroxy” group f. Practice – Common names – Aldehydes a. 1C formaldehyde b. 2c acetaldehyde c. 3C propionaldehyde d. 4C butyrlaldehyde V. VI. Naming Ketones – page 127 (do practice exercise) a. Locate longest C chain containing the ketone group b. Change ending of name from “e” to “one: i. Propanone – 3 C ketone c. Number the chain, so the carbonyl group has the lowest possible number, put this number in front of the name of the parent chain d. Locate substituents, list in alphabetical order i. Comments – in a ringed ketone, the ketone C is C #1, no number is needed ii. VII. Alternate naming convention and Common names a. Name 2 alkyl groups on the 2 sides of the ketone in alphabetical order i. Ethyl methyl ketone – comment on MEK b. 3C ketone – acetone c. Methyl phenyl ketone - acetophenone VIII. Isomers a. Positional – for ketones b. Skeletal – for both c. Functional group isomers – when a ketone and aldehyde are isomers of each other i. 4 C ketone and a 4C aldehyde IX. X. Interesting Aldehydes/Ketones – see pages 129-132 Physical Properties a. State of matter i. Aldehydes 1. 1-2 C gases 2. 3-11 C liquids, 3. 12 C and larger are solids ii. Ketones 1. Small ketones are liquids b. Boiling Point i. Carbonyl group is not as polar as the –OH group, so BP is lower than that of alcohol with same # of carbons 1. Dipole-dipole forces, not H bonds between carbonyl groups ii. Carbonyl group is polar, so BP is higher than that of alkane with similar molar mass – see page 132 c. Water solubility i. Cannot H bond to each other, but can H bond to water ii. Solubility decreases as C chain gets longer – see page 133 d. Odor i. small aldehydes have strong, pungent, unpleasant odors ii. larger aldehydes have pleasant odors – benzaldehyde iii. most ketones have pleasant odors XI. Reactions 1. Synthesis of aldehydes and ketones – oxide alcohols -- 10 alcohol ---(O)--- --20 alcohol ---(O) - aldehyde ketone 2. Oxidation of Aldehydes (and Ketones) a. aldehydes are easily oxidized to form carboxylic acids -- aldehyde --(O) --- carboxylic acid -- even oxygen in the air can oxidize many aldehydes b. Mild oxidizing agents are used to test for aldehydes – react with ald, but not alcohols 1. Tollen’s Test – aka silver mirror test Reagent: Ag+1 in the presence of NH3 and water and heat 2. Benedict’s test Reagent: Cu+2 in basic solution with heat ? Oxidation of Ketones – not possible 3. Reduction of Aldehydes and Ketones Lab Reagent: H2 with Ni catalyst Reverse of ald/ketone synthesis reaction Aldehyde + H2 Ketone + H2 ---Ni 10 alcohol ---Ni 20 alcohol In the body reduce with NADH, H+ and the appropriate enzyme Show for pyruvate lactic acid Simplified into one step…. O CH3-C- COOH 4. Hemiacetal Formation – page 138 --show hemiacetal functional group -- aldehyde/ketone react with an alcohol to form a hemiacetal -- Not a very stable functional group, difficult to isolate UNLESS it’s a cyclic hemiacetal.