Aldehydes and Ketones: Nomenclature & Reactions
advertisement
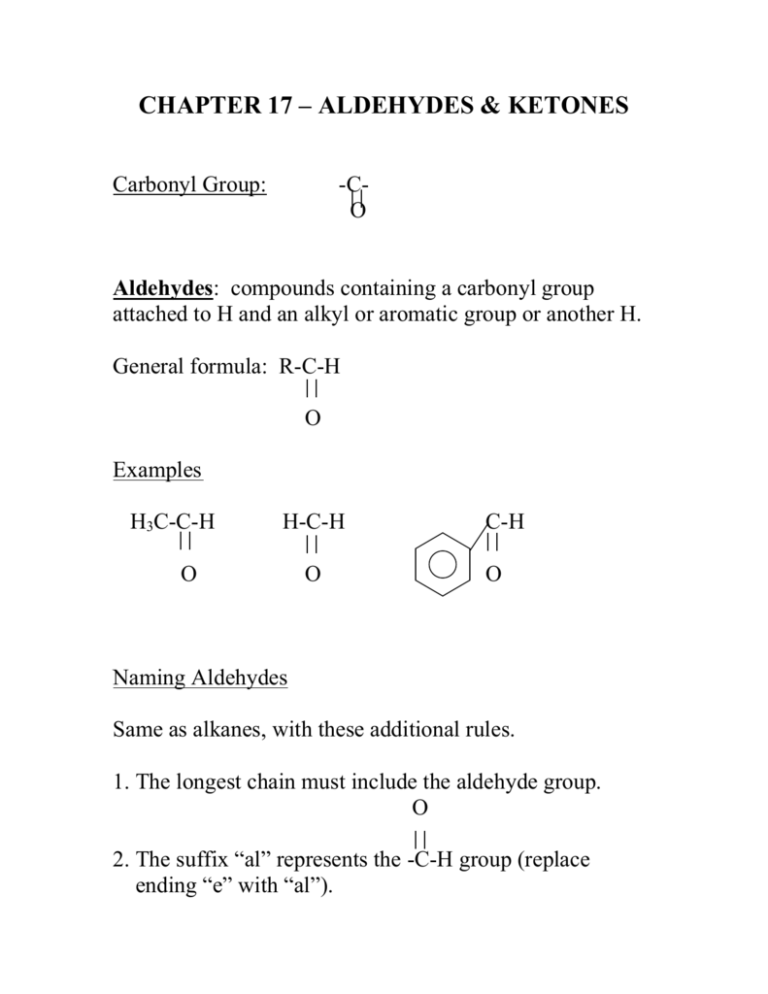
CHAPTER 17 – ALDEHYDES & KETONES Carbonyl Group: -CO Aldehydes: compounds containing a carbonyl group attached to H and an alkyl or aromatic group or another H. General formula: R-C-H O Examples H3C-C-H H-C-H O O C-H O Naming Aldehydes Same as alkanes, with these additional rules. 1. The longest chain must include the aldehyde group. O 2. The suffix “al” represents the -C-H group (replace ending “e” with “al”). Example H O H – C –H H–C–H H methane (formaldehyde) H H H–C – C–H H H Ethane Name this compound: H H H H–C– C–C–C –H H H H O Note: No number is needed in the name to identify the location of the aldehyde group because the aldehyde group is always located at the end of the chain. 3. If groups are attached to the parent, assign the lowest possible number to the carbon of the aldehyde group. O CH3-CH2-CH2-CH-C-H CH2 CH3 Ketones: compounds containing a carbonyl group attached to two alkyl or aromatic groups (or one alkyl & one aromatic). General formula: R-C-R’ O Examples H3C-C-CH3 O C-CH3 O Naming Ketones Same as alkanes, with these additional rules. 1. The longest chain must include the ketone group. O 2. The suffix “one” represents the -C- group (replace ending “e” with “one”). Example H H H H–C–C–C–H H H H propane H O H H–C–C–C–H H H Can you have methanone? 3. When assigning numbers to the carbons in the parent chain, assign the lowest possible number to the carbon attached to the =O Examples Br H3C-C-CH2-CH2-CH3 O H3C-CH2-C-CH2-CH-CH3 O Name these compounds: Cl H3C-C-CH2-CH2-C-CH3 CH3 O CH3 H3C-C-CH2-CH2-C-CH2 CH3 CH3 O H3C-CHCH-CH2-C-H F F O Name the functional groups in this compound. OH OCH3 Vanillin C-H O Oxidation of Aldehydes R-C-H [O] O Aldehyde R-C-O-H O carboxylic acid [O] = oxidizing agent (adds oxygen or loss of hydrogen) (oxygen in air is a mild oxidizing agent) R-C-R’ [O] no reaction O Ketone In lab we can differentiate between an aldehyde and a ketone based on if they react with an oxidizing agent. Silver Mirror Test Tollens’ Reagent: oxidizing agent (contains silver nitrate & ammonia in water) R-C-H + 2Ag(NH3)2+ O Aldehyde 3[OH-] R-C-OH + 2Ag + 4NH3 O carboxylic acid silver mirror Benedict’s Test Benedict’s Solution: oxidizing agent (contains Cu2+ ions, in a basic solution; blue color) R-C-H + 2Cu2+ O Aldehyde [O] R-C-O- + Cu2O O carboxylic acid orange/red ppt Positive test for an aldehyde: blue color disappears & red/orange precipitate forms. Complete the following reactions by drawing the structural formula of the organic product formed in the reaction: O CH3-CH2-CH2-CH2-C-H + Benedict's sol’n O CH3-CH2-CH2-C-CH3 + Benedict's sol’n 4. Aldehyde or ketone groups take precedence over other groups (i.e. alkene, alkyne, alcohol). H2C=CH-CH2-C-CH3 O