The Launching of Blockbuster Drugs During the Twentieth Century
advertisement

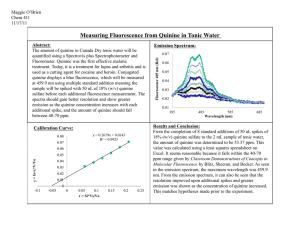
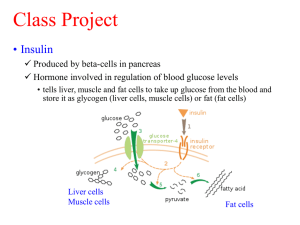
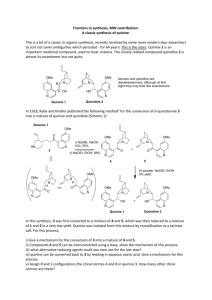
A Short History of the Discovery and Development of Modern Pharmaceuticals Isolation of pure substances • The development of modern pharmacotherapy begins with the isolation of pure substances from plants, through the use of solvent extraction at the beginning of the 19th century Solvent Extraction • German chemist (1747) Andreas Marggraf observed that brandy could extract a crystalline substance (sucrose) from beetroot Solvent Extraction • Swedish chemist Carl Wilhelm Scheele (17421786), applied solvent extraction to the isolation of acids from plant juices • He isolated citric, malic, oxalic, and gallic acids in the 1780s. Where are we today? • Solvent extraction continues to be a valuable part of today’s isolation armamentarium, augmented by column chromatography. Opium Plant • Opium plant (Papaver somniferum) was used in ancient civilizations (4000-1000 BCE) for various medicinal purposes and in religious ceremonies The Latex of the Poppy • Opium is produced by drying the latex that exudes from the capsules of the poppy. • Around 1805-1820, a number of individuals simultaneously reported the isolation of a crystalline, alkaline material from the poppy latex. • Friedrich Wilhelm Serturner (1783-1841) showed in 1811 that administration to dogs made the animals sleepy. • However, the true power of morphine as a painrelieving agent only became apparent after the invention of the hypodermic syringe by Alexander Wood in 1853. • Morphine found great utility in the civil war, but, since ideal dosing was not established, also caused many deaths. • Additionally, many addicts were created (and much quackery practiced). • Even before the structure of the drug was known, a more addictive derivative was generated by simply treating morphine with acetic anhydride, to produce diacetylmorphine, or diamorphine. • The addictiveness of the new drug, heroin, was not immediately appreciated, and it was marketed by Bayer Pharmaceutical. • The chemical structure of morphine was not established till 1923, when J. Masson Gulland and Robert Robinson succeeded in deciphering it. Where are we today? • Morphine, and related opioid alkaloids, remain the most effective method for the treatment of severe pain. It is known that the action of morphine is due to its agonism of the mu (m) opioid receptor in the CNS. • Shown above is an irreversible antagonist of this receptor (structurally related to morphine) • Crystallographic studies of the G-protein coupled receptors (GPCRs) has been difficult, since they are localized in membranes. • Although there has been some recent success Cinchona Bark • As early as the 17th century, the Jesuits in South America were using the bark of the cinchona tree as a cure for malaria. • The cure was brought to Europe, but was not immediately accepted. • Robert Talbor, a London apothecary published a small book called A Rational Account of the Cause and Cure of Agues, and eventually used the drug to cure several royals, including King Charles II. • Pierre Joseph Pelletier and Joseph Bienaimé Caventou succeeded in isolating the active principle, of the cinchona, quinine, in 1820. • The purified quinine was much superior to the nauseating unpalatable cinchona powder and thus immensely successful. • By 1826, Pelletier and Caventou had opened two factories where they were processing 150,000 kg of cinchona bark yearly, yielding around 3600 kg of quinine sulfate. Quinine General structure of heterocycle quinoline • In 1907, Paul Rabe proposed the correct structure of quinine (Rabe, P.; Ackerman, E.; Schneider, W. Ber. 1907, 40, 3655), which was later confirmed by a total synthesis by Robert B. Woodward. • The formal synthesis of quinine, published by R. B. Woodward and W. E. Doering was the launch of Woodward’s career as a master of the art of organic synthesis, and eventually led to a Nobel Prize. Where are we today? Mefloquine Chloroquine • While quinine itself is no longer commonly used (due to the evolution of parasitic resistance), several structurally related derivatives are still commonly employed, both prophylactically and for treatment of the disease. 1900-1910 Drug Name: Epinephrine Trade Name: Adrenalin® (Parke, Davis & Company) Use: Hormone (vasopressor, stimulates heart muscle) Use: Treatment of cardiac arrest, treatment of anaphylaxis, treatment of sepsis, can be mixed with injectable forms of local anesthetics Jokichi Takamine was able to precipitate the free base of adrenaline from aqueous solution by addition of aqueous ammonia solution to raise the pH He had developed a collaboration with the pharmaceutical company Parke, Davis and Company. Where are we today? Epinephrine is a powerful vasoconstrictor In emergency situations, it can be injected intramuscularly or intravenously to raise blood pressure or relieve anaphylaxis in the case of severe allergic responses. It is also injected subcutaneously to locally constrict blood vessels and thus prolong the action of local anesthetics in dental work, for example. 1910 Drug Name: arsphenamine Trade Name: Salvarsan Use: antisyphillitic • Some organoarsenic compounds (such as Atoxyl, shown above) had shown activity in curing sleeping sickness, caused by Trypanosoma brucei, transmitted by the tse-tse fly. Paul Ehrlich was told that the causative agent of syphilis, the spirochete Treponema pallidum, was similar to the trypanosome Trypanosoma brucei, and encourage to try organoarsenicals as curative agents. Ehrlich initiated a program to make an extended library of organoarsenic compounds. The 606th compound he made showed activity against Treponema pallidum and became the treatment for syphilis. It was used from 1910 to 1944, when penicillin became the treatment of choice for this disease. 1912 Drug Name: Phenobarbital Trade Name: Luminal Use: Sedative, anticonvulsant Use: Treatment of epilepsy 1864 1902 Barbituric Acid Barbituric acid was first prepared by Adolph von Baeyer in 1864, (on the feast of St. Barbara) but it was not realized that this class of compounds had biological activity until Emil Fischer showed diethylbarbituric acid (Barbital) could put dogs to sleep in 1904. This drug was used to treat insomnia until 1950. Phenobarbital was made by Emil Fischer in 1902, and brought onto the commercial market as a sedative by Bayer in 1912. Where are we today? As sedatives, the barbituates were displaced by the benzodiazepines in the 1950s, but they are still utilized to treat seizures. 1922 Drug Name: Insulin Use: Hormone Use: Treatment of diabetes mellitus • Insulin is a peptide hormone that is secreted by the pancreas. • Insulin regulates the uptake of glucose from the blood. • Insulin was the first hormone to be isolated. In 1969, Dorothy Crowfoot Hodgkin used x-ray crystallography to obtain the first crystal structure of insulin. LINK Dorothy Hodgkin had earlier received the Nobel Prize in Chemistry for her work on the x-ray crystallographic techniques. 1935 In vivo Metabolism Protosil Drug Name: Active form is Sulfanilamide Trade Name: Prontosil Use: Antiinfective Sulfanilamide Before 1935, there was a desperate need for antibiotics as bacterial infection represented a leading cause of death. The sulfa drugs represented the first highly effective antibiotics and saved thousands of lives, including those of Winston Churchill and Franklin Roosevelt Jr. Where are we today? Sulfamethoxazole Although sulfa drugs are very old antibiotics, they are still widely used today. One common combination includes sulfamethoxazole and trimethoprim to form the combination mixture co-trimoxazole. Trimethoprim These two agents inhibit two enzymes that are used in the bacterial biosynthesis of tetrahydrofolate. 1942 Drug Name: Penicillin Use: Antiinfective Where are we today? It is now known that the target of the penicillins are the PBPs (penicillin binding proteins) transpeptidases responsible for crosslinking peptidoglycan strands. 1957 Drug Name: Chlorpromazine Trade Name: Thorazine Use: Antipsychotic Use: Treatment of schizophrenia 1960 Norethynodrel (progestin) Drug Name: Norethynodrel/mestranol Trade Name: Enovid Use: Hormone Use: Birth Control (1st oral contraceptive) Mestranol (estrogen, demethylated in liver) Where are we now? Norethisterone acetate (Progestin) Ethynylestradiol (Estrogen) Combination progestin/estrogen oral contraception pills remain popular, although there are alternatives. Patches, which gradually administer the hormones transdermally, are alternatives to oral formulations. 1967 Drug Name: Propranolol Trade Name: Inderal Use: b-receptor antagonist (beta-blocker) Use: Antihypertensive • Propranolol was developed by James W. Black. • While working for Imperial Chemical Industries, ICI, a British chemical company, Black developed an interest in the way the heart muscle responded to the hormone adrenalin • He went to work to find a way to block the action of adrenalin and developed the first of the ‘beta-blockers’ which block the action of adrenalin at the beta-adrenergic receptors. 1976 Drug Name: Cimetidine Trade Name: Tagamet Use: H2-receptor antagonist Use: Treat heartburn, GERD, etc. 1981 Drug Name: Captopril Trade Name: Capoten Use: Inhibitor of angiotensin converting enzyme Use: Antihypertensive 1987 Drug Name: Fluoxetine Trade Name: Prozac Use: Selective Serotonin Reuptake Inhibitor (SSRI) Use: Antidepressant 1997 Drug Name: Atorvastatin Trade Name: Lipitor Use: HMG-CoA reductase Inhibitor Use: Cholesterol Reduction 1997 Drug Name: Saquinavir Trade Name: Fortovase Use: HIV protease Inhibitor Use: Treat HIV 1998 Drug Name: Sildenafil Trade Name: Viagra Use: Inhibitor of PDE5 Use: Treat erectile disfunction 1998 Drug Name: trastuzumab Trade Name: Herceptin Use: Monoclonal antibody to the HER2 protein Use: Treat breast cancer 1998 Drug Name: Imatinib Trade Name: Gleevec Use: Inhibitor of the ABL tyrosine kinase Use: Treatment of selected cancers Readings http://www.chemheritage.org/discover/chemistry-inhistory/themes/pharmaceuticals/restoring-and-regulating-the-bodysbiochemistry/banting--best--collip--macleod.aspx Bosch, F.; Rosich, L. The Contributions of Paul Ehrlich to Pharmacology: A Tribute on the Occasion of the Centenary of His Nobel Prize, Pharmacology (2008) 82: 171-179. 160. The first sequence. Fred Sanger and insulin By Stretton Antony O W From Genetics (2002), 162(2): 527-32. Black, J. Reflections on drug research British Journal of Pharmacology (2010), 161: 1204-1216. Homework Questions: 1. Explain how Paul Ehrlich’s early fascination with dyes led to his hypothesis of the treatment of disease with a ‘magic bullet’. What is ‘side chain theory’ and why was it important? 2. What did Ehrlich, Domagk, and Janssen share in common, both from a scientific/investigation perspective, and secondly from a character perspective? 3. Explain the underlying scientific importance of Fred Sanger’s successful sequencing of insulin.