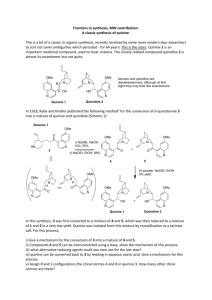
Fluorescence Quadchart
advertisement
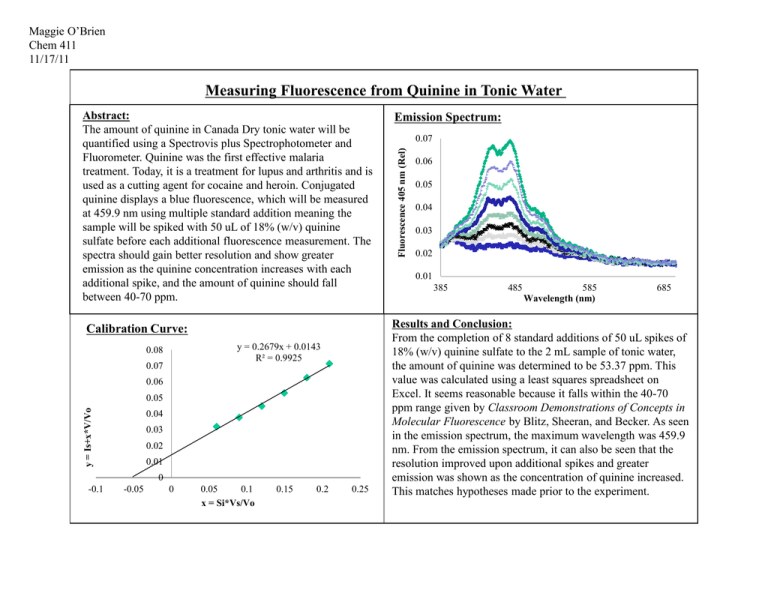
Maggie O’Brien Chem 411 11/17/11 Measuring Fluorescence from Quinine in Tonic Water Calibration Curve: y = 0.2679x + 0.0143 R² = 0.9925 0.08 0.07 0.06 y = Is+x*V/Vo 0.05 0.04 0.03 0.02 0.01 0 -0.1 -0.05 0 0.05 0.1 x = Si*Vs/Vo 0.15 0.2 0.25 Emission Spectrum: 0.07 Fluorescence 405 nm (Rel) Abstract: The amount of quinine in Canada Dry tonic water will be quantified using a Spectrovis plus Spectrophotometer and Fluorometer. Quinine was the first effective malaria treatment. Today, it is a treatment for lupus and arthritis and is used as a cutting agent for cocaine and heroin. Conjugated quinine displays a blue fluorescence, which will be measured at 459.9 nm using multiple standard addition meaning the sample will be spiked with 50 uL of 18% (w/v) quinine sulfate before each additional fluorescence measurement. The spectra should gain better resolution and show greater emission as the quinine concentration increases with each additional spike, and the amount of quinine should fall between 40-70 ppm. 0.06 0.05 0.04 0.03 0.02 0.01 385 485 585 Wavelength (nm) 685 Results and Conclusion: From the completion of 8 standard additions of 50 uL spikes of 18% (w/v) quinine sulfate to the 2 mL sample of tonic water, the amount of quinine was determined to be 53.37 ppm. This value was calculated using a least squares spreadsheet on Excel. It seems reasonable because it falls within the 40-70 ppm range given by Classroom Demonstrations of Concepts in Molecular Fluorescence by Blitz, Sheeran, and Becker. As seen in the emission spectrum, the maximum wavelength was 459.9 nm. From the emission spectrum, it can also be seen that the resolution improved upon additional spikes and greater emission was shown as the concentration of quinine increased. This matches hypotheses made prior to the experiment.