jacobsen quinine ME
advertisement

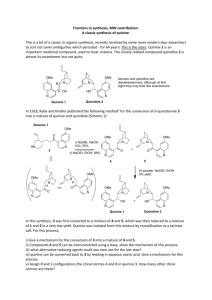
Catalytic Asymmetric Total Syntheses of Quinine and Quinidine Izzat T. Raheem, Steven N. Goodman, and Eric N. Jacobsen J. Am. Chem. Soc. 2004, 126, 3, 706 Presented by Michael Elbaum Dr. Eric N. Jacobsen Born February 22, 1960 B.S. New York University (1982) Ph.D. UCLA Berkeley (1986) Postdoctoral Fellow MIT, Barry K. Sharpless Associate Professor University of Illinois Currently Sheldon Emery Professor of Chemistry, Harvard Development of new methods for organic synthesis with an emphasis on asymmetric catalysis Quinine & Quinidine Cinchona alkaloids have long been known for their medicinal properties; Antipyretic, antimalarial, analgesic and anti-inflammatory Naturally occurs in the bark of cinchona trees Correct connectivity was discovered by Rabe in 1907 First synthesis of quinine from quinotoxine by Rabe and Kindler in 1918 Woodward and Doering synthesizes quinotoxine in 1944 First stereoselective approach used by Uskokovic and Gutzwiller in 1978 First entirely stereoselective total synthesis of quinine by Stork in 2000 Quinine & Quinidine Initial Approach Initial Approach Fragment A Synthesis: Honer-Wadsworth-Emmons (HWE) Reversible reaction allows Thermodynamically Stable, (E) product Fragment A Synthesis: Catalyzed Michael Addition Fragment A Synthesis: Hydrogenation / Lactamization Cis/Trans 1:1.7 converted to 3:1 with: i. LDA, THF, -78o C ii. H2O/THF (5%), -78o C Fragment A Synthesis: Wittig Olefination Fragment A Synthesis: Alkylation Fragment B Synthesis Suzuki Coupling of A & B Ligand Gift from Buchwald Suzuki Cross-Coupling Sharpless Asymmetric Dihydroxylation ADmix-Beta = DHQD Admix-Alpha = DHQ Epoxidation CBz Removal / Intramolecular Sn2 Conclusion 5% total yield Longest linear step is 13 Quinine remains a target for total synthesis