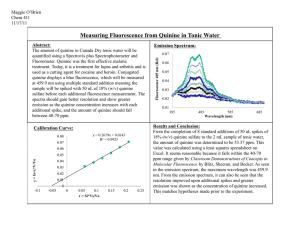
Frontiers in synthesis, MW contribution:
advertisement
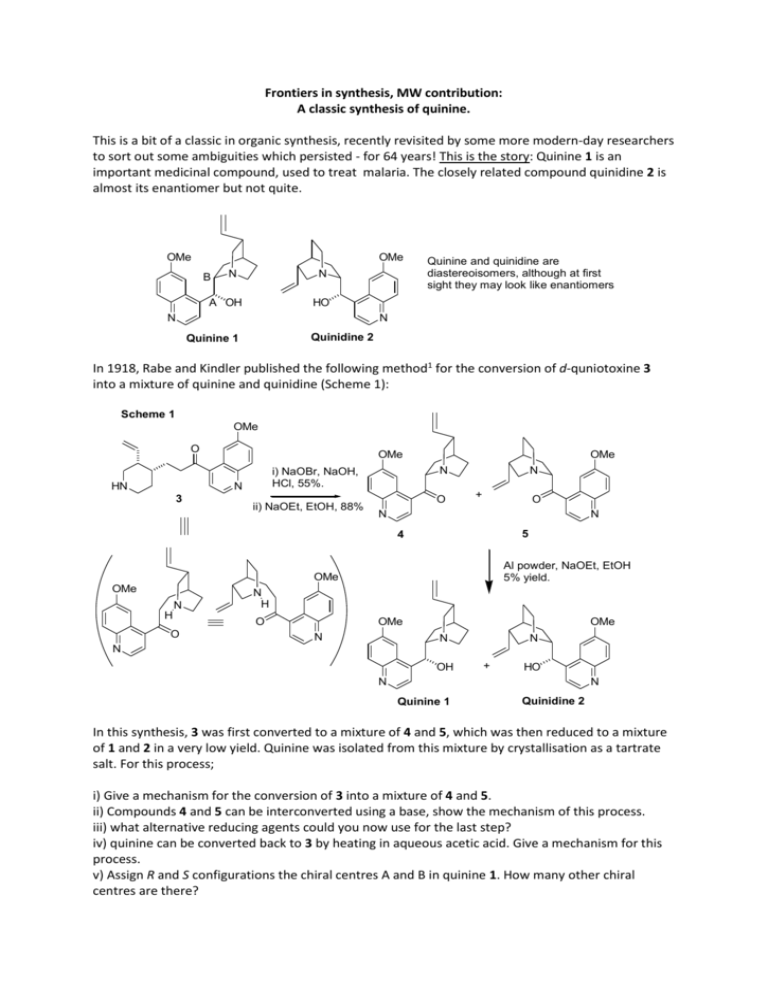
Frontiers in synthesis, MW contribution: A classic synthesis of quinine. This is a bit of a classic in organic synthesis, recently revisited by some more modern-day researchers to sort out some ambiguities which persisted - for 64 years! This is the story: Quinine 1 is an important medicinal compound, used to treat malaria. The closely related compound quinidine 2 is almost its enantiomer but not quite. OMe OMe B N N A OH Quinine and quinidine are diastereoisomers, although at first sight they may look like enantiomers HO N N Quinidine 2 Quinine 1 In 1918, Rabe and Kindler published the following method1 for the conversion of d-quniotoxine 3 into a mixture of quinine and quinidine (Scheme 1): Scheme 1 OMe O HN OMe 3 N i) NaOBr, NaOH, HCl, 55%. N ii) NaOEt, EtOH, 88% OMe O N + O N N 5 4 Al powder, NaOEt, EtOH 5% yield. OMe OMe N H O N H O OMe N OMe N N N OH + HO N N Quinine 1 Quinidine 2 In this synthesis, 3 was first converted to a mixture of 4 and 5, which was then reduced to a mixture of 1 and 2 in a very low yield. Quinine was isolated from this mixture by crystallisation as a tartrate salt. For this process; i) Give a mechanism for the conversion of 3 into a mixture of 4 and 5. ii) Compounds 4 and 5 can be interconverted using a base, show the mechanism of this process. iii) what alternative reducing agents could you now use for the last step? iv) quinine can be converted back to 3 by heating in aqueous acetic acid. Give a mechanism for this process. v) Assign R and S configurations the chiral centres A and B in quinine 1. How many other chiral centres are there? Continuing the story; In 1944, Woodward and Doering published a ‘formal’ total synthesis of quinine,2 starting from simpler reagents. They completed the synthesis of 3 and then stopped at that stage, since the conversion of 3 to 1 had already been reported.1 However for many years doubt was cast on the original results obtained by Rabe and Kindler,1 because no report of the successful repetition of their synthesis was reported, either by Woodward an Doering or anyone else. This controversy was finally resolved when Smith and Williams published, in 2008, a successful reproduction of the 1918 results, using reagents available at that time.3 The story makes for very interesting reading. Here are some steps from the Woodward and Doering synthesis (Schemes 2 and 3). The isoquinoline formed in Scheme 2 is the precursor of the piperidine ring compound 10: Scheme 2 OH OH EtO EtO + 6 NH 2 7 O 100oC EtO 76% pure H2SO4 (neat) EtO 0oC->rt N 94% Scheme 3 + N Ph EtO 10 64% several 10 steps N 9 OMe OMe O O 8 OH O i) dry NaOEt EtO N O 11 ii) 6M HCl, reflux 50% for two steps HN N 3 i) Give a mechanism for the conversion of 6 and 7 into 8, and for the conversion of 8 to 9. ii) Provide a mechanism for the conversion of 10 and 11 to 3, bearing in mind that there are multiple steps, and a Claisen condensation is involved. iii) For advanced users - how might you get from 9 to 10? This is a multistep process and quite complex. References (well worth reading if you want to know more). 1) P. Rabe and K. Kindler, Ber. Dtsch. Chem. Ges. 1918, 51, 466-467. 2) (a) R. B. Woodward and W. E. Doering, J. Am. Chem. Soc. 1944, 66, 849. (b) R. B. Woodward and W. E. Doering, J. Am. Chem. Soc. 1945, 67, 860-874. 3) A. C. Smith and R. M. Williams, Angew. Chem. Int. Ed. 2008, 47, 1736-1740. Reference 2a is a work of art in the arena of concise writing, covering only three quarters of a page with no diagrams, achieved before NMR and IR were available to follow reactions. It really shows what remarkable things can be achieved through meticulous and well thought-out experimentation. There have been other syntheses of quinine since 1944, which you can find through a literature search.