Lesson 2.
advertisement

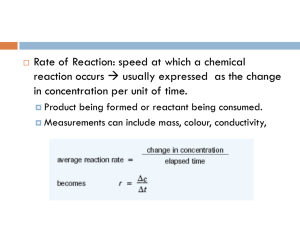
Kinetics Lesson 2 Increasing the Reaction Rate Factors that Affect the Reaction Rate A homogeneous reaction is one where all the reactants are in the same phase. They are Fast due to thorough mixing and lots of collisions! H2(g) + O2(g) → AgNO3(aq) + NaCl(aq) → Factors that Affect the Reaction Rate A homogeneous reaction is one where all the reactants are in the same phase. They are Fast due to thorough mixing and lots of collisions! H2(g) + O2(g) → AgNO3(aq) + NaCl(aq) → same- only look at reactants! Factors that Affect the Reaction Rate A heterogeneous reaction is one where the reactants are in two or more different phases. They are Slow due to poor mixing and fewer collisions. Zn(s) + 2HCl(aq) → Fe(s) + O2(g) → Al(s) + I2(s) → Factors that Affect the Reaction Rate A heterogeneous reaction is one where the reactants are in two or more different phases. They are Slow due to poor mixing and fewer collisions. Zn(s) + 2HCl(aq) → Fe(s) + O2(g) → Al(s) + I2(s) → Ca(s) + H2O(l) → Two solids don’t mix! Factors that Affect the Reaction Rate A heterogeneous reaction is one where the reactants are in two or more different phases. They are Slow due to poor mixing and fewer collisions. Zn(s) + 2HCl(aq) → Fe(s) + O2(g) → Al(s) + I2(s) → Ca(s) + H2O(l) → Different Two solids don’t mix! Factors that Affect the Reaction Rate Mixing can be improved by increasing the surface area. Increasing the surface area can increase the rate of a heterogeneous reaction solids only! Low surface area (slow) Higher surface area (fast) A lump of C burns Powdered carbon explodes A log doesn’t light Kindling lights Factors that Increase the Reaction Rate of Homogeneous and Heterogeneous Reactions 1. Increasing the temperature 2. Increasing a reactant concentration (aq) or (g) 3. Adding a catalyst (an inhibitor decreases the rate) 4. Changing the nature of the reactant (changing a reactant for another that is more reactive) Movie 5. Increasing the pressure of a gaseous reactant Factors that Increase the Reaction Rate of Heterogeneous Reactions Only 6. Increasing the surface area for a heterogeneous reactions only (solid) 7. Agitation (stirring or fanning) for heterogeneous reactions only. What reactions are faster than others? 1. Homo’s are faster than hetero’s. 2. Fastest (aq) 3. (g) (l) Slowest (s) of the reactants Simple ionic equations are the fastest because there are no bonds to break (double replacement). Fast AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) Fast Ag+ + NO3- + Na+ + Cl- → AgCl(s) + Na+ + NO3Fast Ag+ + Cl- → AgCl(s) All three are simple ionic! Describe each Pair of reactions as faster or slower. 8Cu(s) + S8(s) → 8CuS(s) 2NaClO3(aq) + F2(aq) → 2NaF(aq) + 3O2(g) + Cl2(g) Describe each Pair of reactions as faster or slower. 8Cu(s) + S8(s) → 8CuS(s) Hetero 2NaClO3(aq) + F2(aq) → 2NaF(aq) + 3O2(g) + Cl2(g) Homos are faster- more collisions Describe each Pair of reactions as faster or slower. AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) 2NaClO3(aq) + F2(aq) → 2NaF(aq) + 3O2(g) + Cl2(g) Describe each Pair of reactions as faster or slower. AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) 2NaClO3(aq) + F2(aq) → 2NaF(aq) + 3O2(g) + Cl2(g) Hmmmm.…………both homo…… Describe each Pair of reactions as faster or slower. AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq) Simple ionic- double replacement- fastest! 2NaClO3(aq) + F2(aq) → 2NaF(aq) + 3O2(g) + Cl2(g) Describe each Pair of reactions as faster or slower. 3Ca(s) + 2P(s) → Ca3P2(s) I2(g) + H2(g) → 2HI(g) Describe each Pair of reactions as faster or slower. 3Ca(s) + 2P(s) → Ca3P2(s)) Hetero- both solids- slow- less reactant collisions! I2(g) + H2(g) → 2HI(g) Homo- both aqueous- fast- more reactant collisions! State five ways to increase the rate of this reaction. Mg(s) + 2HCl(aq) → H2(g) + MgCl2(aq) 1. Increase temperature 2. Increase [HCl] 3. Add a catalyst 4. Increase surface area of Mg 5. Agitate State three ways to increase the rate of this reaction. 2H2O2(aq) → O2(g) + 2H2O(l) 1. Increase temperature 2. Increase [H2O2] 3. Add a catalyst State six ways to increase the rate of the following reaction. 4Fe(s) + 3O2(g) → 2Fe2O3(s) 1. Increase temperature 2. Increase [O2] 3. Add a catalyst 4. Increase the pressure of O2 5. Increase surface area of Fe 6. Agitate Demonstration Sucrose + potassium chlorate = rocket fuel H2SO4 C12H22O11(s) + 12O2(g) → 12CO2 + 11H2O + energy Let’s increase the rate by: 1. 2. 3. 4. Adding the catalyst H2SO4 Increasing the [O2] to 100 % by adding KClO4 Allowing the temperature to increase to 2000 oC (exo) Increasing the surface area of C12H22O11 (powdered) It should be Fast- Movie