Rate of Reaction: Definition & Measurement
advertisement

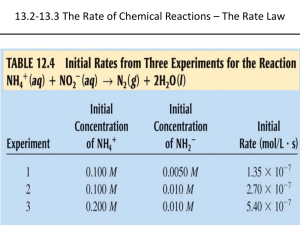
Rate of Reaction: speed at which a chemical reaction occurs usually expressed as the change in concentration per unit of time. Product being formed or reactant being consumed. Measurements can include mass, colour, conductivity, volume, and pressure. RATES OF REACTION (CHAPTER 6) AVERAGE AND INSTANTANEOUS RATES OF REACTIONS Reaction rates are not usually constant change with time. If we use former equation, will only get an average rate no instantaneous. Instantaneous rate: rate of reaction at a particular time. Do so by drawing tangent line at the point in time on a concentration-time graph. Slope of the tangent is the instantaneous rate of the reaction. Go over the ThoughtLab on page 270. There may be something like this on the exam! Reaction Rates in Terms of Products and Reactants Just do ratios! PPs, page 272, #1-4 6.2- The Rate Law: Reactant Concentration and Rate In this section, we will study the reaction rates that are not affected by the concentration of the products. Rate of reaction generally increases when the concentrations of the reactants increase. RATE LAW Rate at which a reaction occurs. k: rate constant: different for each reaction at a given temperature. m & n: must be determined by experiment. If m or n = 1, reaction is in ‘first order in this reactant.’ If m or n = 2, reaction is in ‘second order in this reactant.’ (m + n) overall reaction order. THE RATE CONSTANT, k Indicates the speed of a reaction. As reaction proceeds, the reaction rate decreases because the concentration of the reactants decrease, but value of k remains constant. Must use specific ks for each temperature. DETERMINING A RATE CONSTANT Be aware: the units associated with k may change depending on the order of the equation! As long as you calculate using your units and crossing off where applicable, you should end up with the right units! PG. 7&8 SR: #1, 3, 5 6.3- Theories of Reaction Rates 5 BASIC FACTORS AFFECT REACTION RATES: chemical nature of reactants concentration of reactants temperature presence of a catalyst surface area COLLISION THEORY: IN ORDER FOR A REACTION TO OCCUR, REACTING PARTICLES MUST COLLIDE WITH ONE ANOTHER. CHEMICAL NATURE OF REACTANTS different elements react at different rates e.g Au and Ag react slowly in air while Na and K react so quickly in air that they are rarely found naturally in their elemental state similar elements in the same group tend to react similarly but at different rates e.g. Zn, Fe and Pb all react with HCl acid to produce H2 gas, even when all the other conditions are the same, the rates of reaction are different e.g. activity series in homogeneous systems, such as reactions in aqueous solution, most reactions with monatomic ions (e.g. Ag+ and Cl-) are extremely fast, while reactions of molecular substances are often slower (e.g. glucose (C6H12O6) and iron (II) ions (Fe2+) react with purple permanganate (MMnO4-) ions very visibly: glucose reacts slowly when compared to the reaction with Fe2+ CONCENTRATION/SURFACE AREA concentrated HCl vs. vinegar (acetic acid) or human stomach acid shows how different concentrations can cause different reaction rates experiments suggest that if the initial concentration of a reactant is increased, than the reaction rate generally increases e.g. when one adds a metal to acid, a higher initial concentration of acid increase the rate of gas production implies “concentrated is better & faster” HOW DOES THIS RELATE TO THE COLLISION THEORY? SURFACE AREA? TEMPERATURE mixing ingredients for a cake and nothing happens until the cake is placed in an oven cooking uses increased temperature to make changes in food happen more quickly paint dries faster when the temperature of the system increases, etc. rule of thumb is that around SATP a 10°C rise in temperature often doubles or triples the rate of a chemical reaction lowering the temperature can likewise lower the rate of a chemical reaction, useful for food storage humans apply cold substances to burned skin to slow unwanted physiological reactions (help minimize the effect of the burn) Read pg. 290 – 294 and do SP on page 293. PPs 13-15 SR #1, 3