Chemical Kinetics: Reaction Rates, Mechanisms, and Factors
advertisement

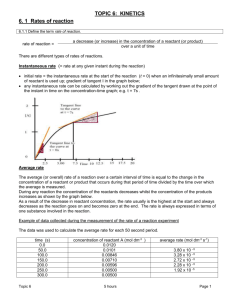
Ch. 6 Learning Goals: Kinetics • Calculate average and instantaneous rates of reaction from data in tables and graphs. • Sketch graphs of [R] vs. time and [P] vs. time. • Use stoichiometric relationships to calculate rates of consumption and production. • Propose methods of measuring the rate of a reaction. • Explain, using collision theory and potential energy diagrams, how factors such as temperature, the surface area of the reactants, the nature of the reactants, the addition of catalysts, and the concentration of the solution control the rate of a chemical reaction. • Draw and describe simple potential energy diagrams of chemical reactions (e.g., the relationships between the relative energies of reactants and products and the activation energy of the reaction). • Explain the significance of the Maxwell-Boltzmann distribution for two different temperatures. • Calculate the rate law, including the value and units for k. • Use the rate law to calculate the rate of a reaction. • Explain how the rate of a reaction is determined by the series of elementary steps that make up the overall reaction mechanism. • Identify the rate determining step and relate the rate of the overall reaction to the rate law of the RDS. • Use appropriate terminology related to rates of reaction, including, but not limited to: activation energy, endothermic, exothermic, potential energy diagram, orientation, reaction rate, elementary step, reaction mechanism, reaction intermediate, rate determining step • Conduct an experiment to gather data for the purpose of determining the order of a reaction with respect to a particular reactant. • Plan and conduct an inquiry to determine how various factors (e.g., change in temperature, addition of a catalyst, increase in surface area of a solid reactant) affect the rate of a chemical reaction. Ch. 6: Chemical Kinetics The fact that a reaction occurs tells us nothing about the rate at which it occurs. • rate of reaction – the speed at which a chemical reaction occurs • usually expressed as change in concentration of reactants or products over time • r = Δc units? Δt Sketch a graph of c vs. t for [product] Graph for [reactant] vs t? Graphing and Related Analytical Skills • • • • • • pencil, ruler, as large as possible Title concentration (y-axis) time (x-axis) plot pencil dot points draw line of best fit for linear data, smooth curve for non-linear data average rate = slope of secant instantaneous rate = slope of tangent Consider: Mg(s) + 2 HCl(aq) --> H2(g) + MgCl2(aq) The rate of this reaction can be measured by: a) measuring change in conductivity over time (MgCl2(aq)) or colour intensity (spectrophotometer) b) measuring change in pH over time ([H+(aq)]) (pH probe) c) measuring volume and/or pressure of H2(g) over time Stoichiometry and Reaction Rate 2A+3B C + 4D rate of consumption of A -Δ[A] Δt -Δ[A] : Δt rate of production of C Δ[C] Δt Δ[C] = 2 : 1 Δt -½ Δ[A] = -⅓ Δ[B] = Δ[C] = ¼ Δ[D] Δt Δt Δt Δt Try this! • Lab 6.1.1 (p. 390) • Complete a-f HW: • p. 360 #1,2 • p. 361 #1-5 Factors Affecting Reaction Rate 1. 2. 3. 4. Nature of reactant (K is more reactive than Na.) Temperature (RαT i.e. T R) Catalyst (MnO2 R of decomposition of H2O2) Concentration (Skittle burns rapidly in 100% O2(g) compared to air at 20% O2(g)) 5. Surface Area (A crushed antacid tablet reacts quicker than a tablet in one piece.) HW: p. 365 #1-5 Collision Theory and Rate of Reaction • R convert to P by successful collisions b/w R • For a collision to be successful, reactant particles must collide with: – the minimum energy required to produce P particles (Activation Energy) – the proper spatial orientation (geometry) Rate = (frequency)(% of effective collisions) frequency of effective collisions, rate Consider: A + B-C reactants A---B---C A-B + C activated complex products (aka transition state) (bonds partially broken/formed) Temperature of the Reaction System: Maxwell-Boltzmann Distribution Why does T rate? Effect of Temperature as KE, particles collide more often ( frequency) at a higher temperature, more particles have the required Ea than at a lower temperature Effect of Concentration and Surface Area increases frequency of collisions Effect of Chemical Nature of Reactant fewer bonds to be broken = faster rate weak bonds – lower threshold energy lower Ea = faster rate stronger bonds – higher threshold energy higher Ea = slower rate collision geometry may be more difficult to achieve with more complex molecules and ions Effect of a Catalyst provides an alternative energy pathway (lower Ea), thereby % of effective collisions since more particles possess the required Ea Maxwell-Boltzmann Distribution and Catalysts Rate = (frequency) (% of effective collisions) Factors affecting frequency? temperature surface area concentration Factors affecting % of effective collisions? temperature nature of reactants catalyst Homework: • p. 372 #1-5 Concept Check: Label i, ii, iii. Rate Law and Order of Reaction Given: aA + bB products By expt, rate α [A]m[N]n (Rate law) rate = k[A]m[N]n where k is the rate constant m, n indicate the sensitivity of the rate to changes in [A] or [B], not related to coefficients Order of reaction – the exponents in the rate law Ex. Given 2 NO(g) + 2 H2(g) N2(g) + 2 H2O(g) R = k[NO(g)]2[H2(g)] The reaction is 2nd order in NO(g) and 1st order in H2(g). The overall reaction is 3rd order. Ex 1: Given R= 1.1 x 104 M-2s-1 [BrO3-][HSO3-]2, determine the rate when [BrO3-] = 0.0020 M and [HSO3-] = 0.0060 M. Ex 2: Given 2A + B + 3C products The reaction is 1st in [A], 2nd order in [B] and third order overall. What is the affect on the rate if: a) [A] is doubled? b) [B] is tripled? c) [C] is doubled? d) [B] is halved? Relating Reaction Rate to Time (p. 378) Homework • p. 380 #1-5 or p. 382 #1-4 • Print Iodine Clock lab from website. Complete pre-lab. Pre-lab: Title, Question, Hypothesis, Variables, Complete concentration calculations in table, plan graphical analysis. analogy dirty dry dishes clean wet dishes clean wet dishes clean dry dishes dirty dry dishes clean dry dishes Reaction Mechanisms • Reaction mechanism – a series of elementary steps that predict how reactants are converted to products • elementary steps – one-step process in which product particles are (in most cases) the result of collisions b/w 2 reactant particles (bimolecular). Trimolecular collisions are rare. Ex. 2 NO(g) + H2(g) N2(g) + H2O2(g) H2O2(g) + H2(g) 2 H2O(g) 2 NO(g) + 2H2(g) N2(g) + 2 H2O(g) Rate Determining Step – the slowest step in the mechanism (determines the rate of the overall reaction) Ex. Cl2 2 Cl Cl + H2 HCl + H H + Cl2 HCl + Cl Cl + Cl Cl2 Write the overall equation. The rate law of the overall reaction is R=k[Cl2] Which step is the RDS? Homework: • p. 386 #1-3 • p. 387 #1-9 • Unit 3 Summary note