Limiting Reactant
advertisement

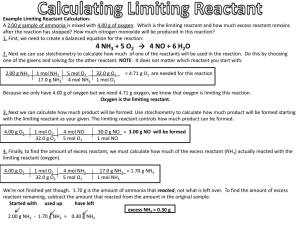
Limiting Reactant A.K.A. Stoichiometry How do we determine which reactant limits the product? One reactant remains while the other is consumed in a reaction Mole ratio will help determine the limiting reaction Need to create a conversion pathway How Do Hot Fudge Sundaes and Limiting Reactants Relate? How Many Sundaes Can You Make? Two! The chocolate syrup limits the recipe! Which reactant is the limiting reactant? Which reactant remains when 6.70 mole Na reacts with 3.2o moles of Cl2 2 Na + Cl22 NaCl Step 1 Moles of reactant Moles of product 2 Na + Cl22 NaCl Step 1 Step 1 Moles of Na Mole Ratio Moles of NaCl 6.70 moles of Na 2 mol NaCl = 6.70 mol NaCl 2 mol Na Moles of Cl2 Mole Ratio Moles of Product 3.20 moles of Cl2 2 mol NaCl = 6.40 mol NaCl 1 mol Cl2 Cl2 is the Limiting Reactant! It’s Your Turn… Limiting Reactant Worksheet Limiting Reactant and Mass-Mass Conversions Which reactant is the limiting reactant when 3.5 g Cu reacts with 6.0 g AgNO3? Cu + AgNO3 Cu(NO3)2 + Ag; balanced? Cu + 2 AgNO3 Cu(NO3)2 + 2 Ag Create a Conversion Pathway Cu + 2 AgNO3 Cu(NO3)2 + 2 Ag We have 3.5 g Cu and 6.0 g AgNO3 Step 1 Mass of Reactant Step 2 Moles of Reactant Step 3 Moles of Product Mass of Product Cu + 2 AgNO3 Cu(NO3)2 + 2 Ag Mass of Cu Molar Mass of Cu Mole Ratio Molar Mass of Ag Mass of Ag 3.5 g Cu 1 mol Cu 2 mol Ag 108 g Ag = 12 g Ag 63.5 g Cu 1 mol Cu 1 mol Ag Mass of AgNO3 Molar Mass of AgNO3 Mole Ratio Molar Mass of Ag Mass of Ag 6.0 g AgNO3 1 mol AgNO3 2 mol Ag 108 g Ag = 3.8 g Ag 169.9 g AgNO3 2 mol AgNO3 1 mol Ag Silver nitrate is the limiting reactant! Homework Read pp. 257-258 Answer problems pg. 263: 45