Kinetics Powerpoint
advertisement

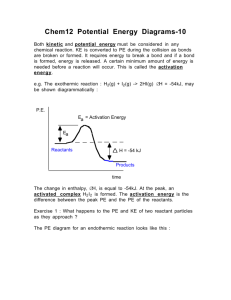
Reactants must collide with proper orientation and sufficient energy Explains what happens once colliding particles react ◦ Transition state is the in-between state when reactants are being converted to products ◦ Kinetic energy is converted to potential energy (think about bouncing a basketball) Kinetic Energy Potential Energy Negative Exothermic reactions- give off heat (products more stable, less potential energy) Endothermic reactions require heat (products less stable than reactants, higher PE) Exothermic Reaction Endothermic Reaction Draw a PE diagram. Include: axes labels, the transition state, activated complex, Ea (forward and reverse), and ΔE. CO reacts with NO2 to form CO2 and NO. The activation energy of the forward reaction is 134 kJ and the ΔE is -226 kJ. Chemical reactions typically occur as a series of steps. This series of steps that make up the overall reaction is called the reaction mechanism. Elementary reactions are a single step in the overall reaction mechanism. ◦ Singular molecular event, such as a simple collision of atoms, molecules or ions. ◦ Cannot be broken down into further simpler steps. For example, the reaction 2NO(g) + O2(g) 2NO2(g) involves a two-step reaction mechanism: ◦ Step 1: NO(g) + O2(g) NO3(g) ◦ Step 2: NO3(g) + NO(g) 2NO2(g) Each step is an elementary reaction, both steps together give the overall reaction mechanism. Notice NO3(g) ◦ Not a product or reactant of overall reaction ◦ Produced then consumed = reaction intermediate Describes the number of reactant particles in an elementary step ◦ Unimolecular = one reactant (CH3)3CBr(aq) (CH3)3C+ + Br- ◦ Bimolecular = 2 reactants come together. Ex: Step 1: NO(g) + O2(g) NO3(g) Step 2: NO3(g) + NO(g) 2NO2(g) ◦ Termolecular = 3 reactants Extremely rare!!! Why? One step in a reaction mechanism is always much slower than the others ◦ Since it is so much slower, it determines the rate of the overall reaction. Hence, this slow step is called the rate determining step ◦ Consider the process of making toast: slow fast Activation energy for rds is always higher ◦ 2 STEP REACTION MEANS 2 TRANSITION STATES AND 1 INTERMEDIATE Increases the rate of a reaction ◦ Homogeneous catalyst: Same phase as reactants ◦ Heterogeneous catalyst: Different phase as reactants Pd Not consumed! ◦ There in beginning and end of reaction ◦ Works by lowering the activation energy Therefore, greater number of collisions have sufficient energy to react Provide alternative reaction mechanism Reaction Rate Concentration Surface Area Catalyst Reactivity Temperature Collision Theory Orientation Rate Expression Exothermic Endothermic Homogeneous Heterogeneous Kinetic Energy Unimolecular Rate Constant Elementary Reaction Mechanism Catalyst Rate Law Reaction Order Molecularity Activation Energy Reverse Reaction Potential Energy Transition State Activated Complex Rate Determining Step Biomolecular Termolecular