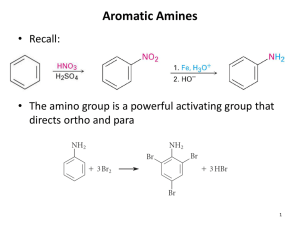
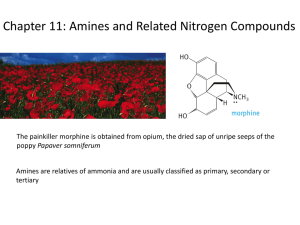
PowerPoint Presentation - Acid -Base Chemistry

Amines
Chemical / Biological / Neurological
Activity
Measures of Basicity
•
•
•
•
• The basicity of amines may be measured and compared by using any of these values:
1) K b
2) p K
3) K a b of conjugate acid
4) p K a of conjugate acid
Basicity Constant (K b
) and pK b
• K b is the equilibrium constant for the reaction:
R
3
N
••
+ H
••
OH
••
+
R
3
N H +
–
••
••
OH
••
K b
=
[R
3
NH + ][HO
–
]
[R
3
N] and p K b
= - log K b
K
a
and pK
a
of Conjugate Acid
• K a is the equilibrium constant for the dissociation of the conjugate acid of the amine:
+
R
3
N H R
3
N
••
+ H +
K a
=
[R
3
N][H + ]
[R
3
NH + ] and p K a
= - log K a
Relationships between acidity and basicity constants
K a
K b
= 10 -14 p K a
+ p K b
= 14
Basicity of Amines in Aqueous Solution
• Amine
• NH
3
• CH
3
CH
2
NH
2
Conj. Acid
NH
4
+
CH
3
CH
2
NH
3
+
CH
3
CH
2
NH
3
+ therefore, CH
3 is a weaker acid than NH
4
+ ;
CH
2
NH
2 is a stronger base than NH
3
.
pK a
9.3
10.8
Effect of Structure on Basicity
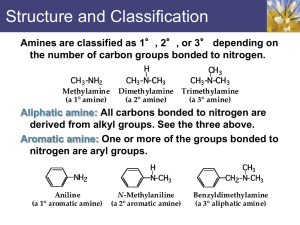
• 1. Alkylamines are slightly stronger bases than ammonia.
• 2. Alkylamines differ very little in basicity.
Basicity of Amines in Aqueous Solution
• Amine Conj. Acid pK a
• NH
3
• CH
3
CH
2
NH
2
NH
4
+
CH
3
CH
2
NH
3
+
• (CH
3
CH
2
)
2
NH (CH
3
CH
2
)
2
NH
2
+
9.3
10.8
10.9
• (CH
3
CH
2
)
3
N (CH
3
CH
2
)
3
NH + 11.1
Notice that the difference separating a primary, secondary, and tertiary amine is only 0.3 pK units.
Effect of Structure on Basicity
• 1. Alkylamines are slightly stronger bases than ammonia.
• 2. Alkylamines differ very little in basicity.
• 3. Arylamines are much weaker bases than ammonia.
Basicity of Amines in Aqueous Solution
• Amine Conj. Acid pK a
• NH
3
• CH
3
CH
2
NH
2
NH
4
+
CH
3
CH
2
NH
3
+
• (CH
3
CH
2
)
2
NH (CH
3
CH
2
)
2
NH
2
+
9.3
10.8
10.9
• (CH
3
CH
2
)
3
N (CH
3
CH
2
)
3
NH + 11.1
• C
6
H
5
NH
2
C
6
H
5
NH
3
+ 4.6
Decreased basicity of arylamines
••
N H
2
••
+ H OH
••
+
N H
3
+
–
••
••
OH
••
• Aniline (reactant) is stabilized by conjugation of nitrogen lone pair with ring p system.
• This stabilization is lost on protonation.
Decreased basicity of arylamines
• Increasing delocalization makes diphenylamine a weaker base than aniline, and triphenylamine a weaker base than diphenylamine.
K b
C
6
H
5
NH
2
3.8 x 10 -10
(C
6
H
5
)
2
NH (C
6 x 10 -14
6
H
5
)
~10
3
N
-19
Effect of Substituents on Basicity of Arylamines
• 1. Alkyl groups on the ring increase basicity, but only slightly (less than 1 p K unit).
• 2. Electron withdrawing groups, especially ortho and/or para to amine group, decrease basicity and can have a large effect.
• X
• H
• CH
3
• CF
3
• O
2
N
X
Basicity of Arylamines
NH
2
X pK b
9.4
8.7
11.5
13.0
pK a
4.6
5.3
2.5
1.0
NH
3
+
p-Nitroaniline
••
O
••
N
+ ••
NH
2
–
••
••
O
••
N
+
–
•• O
••
••
–
•• O
••
••
• Lone pair on amine nitrogen is conjugated with p -nitro group —more delocalized than in aniline itself. Delocalization lost on protonation.
+
NH
2
Effect is Cumulative
• Aniline is 3800 times more basic than p -nitroaniline.
• Aniline is ~1,000,000,000 times more basic than 2,4-dinitroaniline.
Heterocyclic Amines
••
N
H piperidine
K b
= 1.6 x 10 -3
(an alkylamine) is more basic than
N
•• pyridine
K b
= 1.4 x 10 -9
(resembles an arylamine in basicity)
Heterocyclic Amines
••
N
••
N
H is more basic than
K b imidazole
= 1 x 10 -7
N
•• pyridine
K b
= 1.4 x 10 -9
Imidazole
• Which nitrogen is protonated in imidazole?
••
N
••
N
H
H +
H
+
N
••
N
H
H +
••
N N
+
H
H
Imidazole
• Which nitrogen is protonated in imidazole?
(HINT: Resonance is the key.)
••
N
••
N
H
H +
H
+
N
••
N
H
Imidazole
• Protonation in the direction shown gives a stabilized ion.
••
N
••
N
H
H +
H
+
N
••
N
H H
N
••
N
+
H
Question
Which of the following amines is more basic?
• A) B)
• C) D)
Preparation of Amines by Reduction
Preparation of Amines by Reduction
• Almost any nitrogen-containing compound can be reduced to an amine, including:
• azides nitriles nitro-substituted benzene derivatives amides
Synthesis of Amines via Azides
• S
N
2 reaction, followed by reduction, gives a primary alkylamine.
Na N
3
CH
2
CH
2
Br CH
2
CH
2
N
3
Azides may also be reduced by catalytic hydrogenation.
(74%)
1. LiAlH
4
2. H
2
O
CH
2
CH
2
N H
2
(89%)
Question
• What is the product of the reaction shown?
• A)
• C)
B)
D)
Question
• Identify compound C formed in the synthetic sequence below.
• A) ( R )-2-octanamine
• C) ( R )-2-octanol
B) ( S )-2-octanamine
D) octane
Synthesis of Amines via Nitriles
• S
N
2 reaction, followed by reduction, gives a primary alkylamine.
Na C N
CH
3
CH
2
CH
2
CH
2
Br CH
3
CH
2
CH
2
CH
2
C N
(69%)
Nitriles may also be reduced by lithium aluminum hydride.
H
2
(100 atm), Ni
CH
3
CH
2
CH
2
CH
2
C H
2
N H
2
(56%)
Synthesis of Amines via Nitriles
• S
N
2 reaction, followed by reduction, gives a primary alkylamine.
Na C N
CH
3
CH
2
CH
2
CH
2
Br CH
3
CH
2
CH
2
CH
2
C N
(69%)
The reduction also works with cyanohydrins.
H
2
(100 atm), Ni
CH
3
CH
2
CH
2
CH
2
C H
2
N H
2
(56%)
Question
• What is the major organic product of the synthesis shown?
•
•
•
•
A) C
6
H
5
CH
2
CN
B) C
6
H
5
CH
2
CHO
C) C
6
H
5
CH
2
CH
2
NH
2
D) C
6
H
5
CH
2
NH
2
Synthesis of Amines via Nitroarenes
Cl
H N O
3
H
2
SO
4
Nitro groups may also be reduced with tin (Sn)
+ HCl or by catalytic hydrogenation.
Cl
(88-95%)
1. Fe, HCl
2. NaOH
N O
2
Cl N H
2
(95%)
Question
• Which one of the following is produced when m nitroacetophenone is treated with Sn and HCl followed by NaOH?
• A)
• C)
B)
D)
Question
• Starting with benzene, which of the sequences below will produce p -methylaniline as the major product of the reaction?
•
•
•
• A) 1. HNO
3
, H
2
SO
4
; 2. CH
3
Cl, AlCl
3
; 3. Fe, HCl; 4.
NaOH
B) 1. HNO
3
, H
2
SO
4
; 2. Fe, HCl; 3. NaOH; 4. CH
3
Cl,
AlCl
3
C) 1. CH
3
Cl, AlCl
3
; 2. HNO
3
, H
2
SO
4
; 3. Fe, HCl; 4.
NaOH
D) 1. CH
3
Cl, AlCl
3
; 2. HNO
3
, H
2
SO
4
; 3. H
2
Synthesis of Amines via Amides
O
COH
1. SOCl
2
2. (CH
3
)
2
N H
O
C
(86-89%)
Only LiAlH
4 is an appropriate reducing agent for this reaction.
CH
2
N (CH
3
)
2
1. LiAlH
4
2. H
2
O
N (CH
3
)
2
(88%)
Question
• Identify the product of the synthesis shown.
•
•
•
•
LiAlH4
5. H
2
O
A) C
6
H
5
NH
2
B) C
6
H
5
CH=NH
C) C
6
H
5
CH
2
NH
2
D) C
6
H
5
C(=O)NH
2
Preparation and Reactions of Amines
The Gabriel Synthesis of Primary Amines
Question
• What is the product of the Gabriel synthesis shown?
• A) diethyl ether
• B) ethanol
• C) ethyl amine
• D) CH
3
CH
2
NHNH
2
Reductive Amination
Synthesis of Amines via Reductive Amination
In reductive amination, an aldehyde or ketone is subjected to catalytic hydrogenation in the presence of ammonia or an amine.
R fast R
C O + N H
3
C N H + H
2
O
R' R'
• The aldehyde or ketone equilibrates with the imine faster than hydrogenation occurs.
Synthesis of Amines via Reductive Amination
The imine undergoes hydrogenation faster than the aldehyde or ketone. An amine is the product.
R
R'
C O + N H
3
R
R' C
H
N H
2 fast R
R'
C N H + H
2
O
H
2
, Ni
Example: Primary amines give secondary amines
O
CH
3
(CH
2
)
5
CH + H
2
N
NaBH
3
CN or H
2
, Ni ethanol
CH
3
(CH
2
)
5
CH
2
N H via: CH
3
(CH
2
)
5
CH N
Example: Secondary amines give tertiary amines
O
CH
3
CH
2
CH
2
CH +
N
H
H
2
, Ni, ethanol
N
CH
2
CH
2
CH
2
CH
3
(93%)
Question
•
• How would you accomplish the conversion of propanal into N -ethylN -methylpropanamine?
• A) NH
3
, NaBH
3
CN; CH
3
I; CH
3
CH
2
I
• B) CH
3
NH
2
, NaBH
3
CN; CH
3
COCl, pyridine; LiAlH
4
;
H
2
O
• C) CrO
3
, H
2
SO
4
; SOCl
2
, pyridine; 2 equiv CH
3
NH
2
;
CH
3
I
• D) CH
3
CH
2
NH
2
, H
2
, Ni; (CH
3
CO)
2
O, pyridine;
NaBH
4
Quarternary Amines Can Undergo an
E
2
Elimination Reaction
The Hofmann Elimination
The Hofmann Elimination
• a quaternary ammonium hydroxide is the reactant and an alkene is the product
• is an anti elimination
• the leaving group is a trialkylamine
• the regioselectivity is opposite to the Zaitsev rule.
Quaternary Ammonium Hydroxides are prepared by treating quaternary ammmonium halides with moist silver oxide
CH
2
N (CH
3
)
3
I
–
Ag
2
O H
2
O, CH
3
OH
+
CH
2
N (CH
3
)
3
HO
–
Regioselectivity
Elimination occurs in the direction that gives the less-substituted double bond. This is called the Hofmann rule.
CH
3
CHCH
2
CH
3
+ N (CH
3
)
3
HO
– heat
H
2
C CHCH
2
CH
3
(95%)
+
CH
3
CH CHCH
3
(5%)
Regioselectivity
CH
3
CH
2
H
H
H
C
H
H
+ N (CH
3
)
3
H
C
CH
3
CH
2
H major product largest group is between two H atoms
Regioselectivity
H
H
CH
3
CH
3
+ N (CH
3
)
3
H largest group is between an
H atom and a methyl group
CH
3
C
H
C
H CH
3 minor product
Nitrosation of Arylamines
Nitrosation of Primary Arylamines
• Gives aryl diazonium ions.
• Aryl diazonium ions are much more stable than alkyl diazonium ions.
• Most aryl diazonium ions are stable under the conditions of their formation (0-10 °C).
+
R N N fast
R
+
+ N
2
+
Ar N N slow
Ar
+
+ N
2
Synthetic Origin of Aryl Diazonium Salts
Ar H
Ar N O
2
Ar N H
2
+
Ar N N
Synthetic Transformations of Aryl Diazonium Salts
Transformations of Aryl Diazonium Salts
Ar CN
H
3
PO
2
Ar Cl
Cu Cl or
Cu Br
Ar Br
CuCN, heat
Ar F heat
+
Ar N N
HBF
4
/ heat
K I
Ar H
H
2
O , heat
Ar I
Ar O H
Question
• Identify the product isolated from the reaction of
H
2
SO
4 pnitroaniline with NaNO followed by the addition of potassium iodide (KI).
2 in
• A) nitrobenzene
• B) p-iodoaniline
• C) p-iodonitrobenzene
• D) p-diiodonitrobenzene
Alkaloids
Alkaloids: Naturally Occuring Bases
Nitrogen Heterocycles ibogaine
Amines & Neurotransmitters
R C H
2
C H
2
N H
2
H O C H
2
C H
2
N H
2
H O
C H
3
O
H
3
C O C H
2
C H
2
N H
2
H
3
C O
R -ethylamine dopamine mescaline
HO
CH
2
CH
2
NH
2
CH
3
O
CH
3
O
N
H
CH
2
CH
2
NH
2
CH
3
O
N
H
CH
N
H
2
CH
2
O
NHCCH
3
Serotonin --------- Melatonin
HOCH
2
CH
2
CH
3
N CH
CH
3
3
HO
HO
CH
3
O
COCH
2
CH
2
CH
3
N CH
3
CH
3
OH
CHCH
2
NHCH
3
Acetylcholine
Epinephrine (Adrenaline)
Cathecols: epinephrine & mdma http://faculty.washington.edu/chudler/mdma.html
Principal sympathomimetic adrenal hormone
& a controlled substance
Drug Uptake:
Rank from slowest to fastest.
a) injection; b) ingestion; c) inhalation; d) snorting
A) a<b<c<d
C) b<d<a<c
B) c<a<d<b
D) d<b<c<a
Drug Uptake:
Rank from slowest to fastest.
a) injection; b) ingestion; c) inhalation; d) snorting
A) a<b<c<d
C) b<d<a<c
B) c<a<d<b
D) d<b<c<a
http://www%2Drci.rutgers.edu/%7Elwh/drugs/ http://web.indstate.edu/thcme/mwking/nerves.html#table http://www.sfn.org/briefings/addiction.html
http://faculty.washington.edu/chudler/amp.html
morphine ibogaine
LSD