Amines - hisham
advertisement

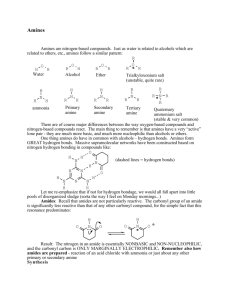
Functional Group Analysis Functional Group Analysis • Classical methods of analysis (wet chemical analysis) analyte + reagent = color or ppt. Main steps: 1. sampling 2. field sample pretreatment 3. Laboratory tretment 4. Laboratory assay 5. Calculation 6. Result presentation Instrumental methods Involve the use of instrument They are: 1. Spectral 2. Electro-analytical 3. Separatory Classical qualitative analysis Classical quantitative analysis Classical qualitative analysis To deduce the identity of the analyte Add reagents that chosen one or single class of chemical compounds (selectivity ) Form ppt. or a gas or color with reagent Other measurements as b.p, m.p, , density …. Examples: addition of Br2 to identify the C=C…. Reaction used to identify the organic molecules (condensation, diazodization, diazocoupling….) Classical quantitative analysis Volumetric analysis (titrimetric analysis) : acid – base , redox , complexometric, preciptmetry that give change in colour with indicator at the e.p at certain volume of titrant Gravimetric analysis: form ppt. with analyte , filtred, dried and weighed Examples of functional group analysis - OH 1. Acylation (esterification) 2. Non-aqueous titration 3. Bromination 4. Periodic acid oxidation 5. spectrophotometry - C=O 1. Oximation 2. Hydrazone formation 3. Condensation with active methylene Amines 1. Acid – base titration 2. Condensation (1ry amine) with carbonyl compounds 3. Reaction with 9-chloroacridine 4. With formaldehyde/acetone 5. Diazotization and diazocoupling 6. Charge – transfer 7. Ion-pair complexation Thiol 1. AgNO3 2. Br2 - COOH 1. Acid – base titration 2. Saponification 3. Reaction with ferric hydroxamete Others 1. Zeissel method for OR/N-alkyl group 2. Drug with reducing activity 3. Binary complex formation 4. Ternary complexation reaction Hydroxyl group –OH alcoholic, glycols, enols and phenols 1. Acylation (esterification): acid alcohol ester Acid chloride or acid anhydride is used as they more potant than the parent acid 2. Non aqueous titration Phenols and enols are weak acidic compounds can dermined by titration with NaOCH3 in DMF and thymol blue as indicator 3. Bromination of phenolic compounds The excess bromine liberate iodine from KI and titrated against NaS2O3 using starch as indicator Aromatic amines undergo the same reaction so interfere in this analysis. Also unsaturated compounds interfere by addition of bromine 4. Periodic acid oxidation of 1,2- glycol Propylene glycol: CH3CHOHCH2OH + 2 HIO4 = CH3CHO + HCHO + HIO3 + H2O excess KI I 2 titrated using Na2SO4 Glycerol NaOH CH2OH-CHOH-CH2OH + 2HIO4 = 2CH2O + HCOOH + 2HIO3 + H2O HCOOH + NaOH = HCHO + H2O 1 glycerol = 1 NaOH using bromocresol green as ind. (dis. Glycol) 5. Spectrophotometry 1. By coupling with Diazonium salt (azo dye) 2. Nitrosation 3. Coupling with antipyrine in presence of oxidizing agent Carbonyl group –C=O aldehyde/ ketons 1. Oximation Oximes can be formed by the action of hydroxylamine on aldehydes or ketones 2. Hydrazone formation 3. Condensation with Active methylene group • Aldehyde and ketones form highly conjugated reaction product with these reagents Knoevenagel condensation Chlorobenzaldehyde Yellow colour product Carboxylic acid and their derivative - COOH RCOOH RCOOR’ RCONHR’ RCONHCOR’ (imide) (RCO)2O RCOCl imide Carboxylic-acid-anhydride Acyl-chloride Carboxylic acid Ester Amides 1. Acid – base titration 2. Saponification 3. Ferric hydroxamate (Esterification and the hydroxamic acid test) Esters react with hydroxylamine in basic solution to form hydroxamic acids, which in turn react with ferric chloride in acidic solution to form bluish red ferric hydroxamate Amines – NH 1ry amine 1. Condensation with carbonyl compounds 2. Reaction with 9-chloroacridine 3. formaldehyde/acetone 4. Diazotization and diazocoupling 2nd amine CS2 + KOH + Cu 3rd amine 1. Charge – transfer 2. Ion-pair complexation 1. Primary amine: 1. Condensation with carbonyl compound Schiff’s base (spectrophotometry) 2. Reaction with 9- chloroacridine: 3. Reaction with formaldehyde/ acetone • Hantzsch pyridine synthesis Reaction with dansyl chloride (dansylation) Fluorescent product 4. Diazotization and diazo-coupling 2. Secondary amine: 3. Tertiary amines • Charge transfer method: Complexes between , Electron donor (amines and their derivatives): electron acceptor (chloranil, p-chloranailic acid, iodine ….) Acceptor Donner • Ion – pair complex B+ + A - AB Amines famotidine methyl orange, bromothymol blue , salicylate …. Thiol, sulphide, disulphide -SH , RSR, RSSR RSH + AgNO3 = RSAg + HNO3 RSR + Br2 = RSOR + HBr RS-SR + Br2 = 2 RSO2Br +HBr Miscellaneous 1. Zeisel’s method (for Alkoxy OR, and N-Alkyl): alkoxy group is treated with hydrogen iodide and the alkyl halide formed is further treated with silver nitrate to precipitate silver iodide, collected and weighed CH3OC2H5 + 2 HI = CH3I + C2H5OH Volatile Methyl or ethyl iodide only piroxicam indomethacin 2. Drugs with reducing activity: When 1,10-phenanthroline is added to even a dilute solution of iron(II), the highly colored complex ion [Fe(phen)3]2+ is produced. 3. Binary complex: Drugs containing NH2 and - COOH form complex with Metals extractable with organic solvent . Example mefenamic and flufenamic acid contain NH and COOH form blue colour complex with copper in alkaline medium and purple with iron in acid medium 4. Ternary complex formation (Ln Mx Sy) Used in spectrophotometric analysis More sensitive than the birany complex The complex of SCN, Cr(III), Zn(II), Co(II), Fe(III) are widely used. metal Ligand Ofloxacine Drug (Ln Mx Sy)