AMINES, AMIDES and ANILINE
advertisement

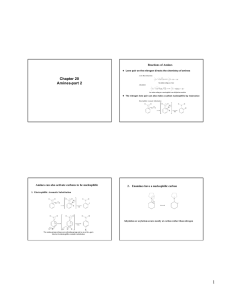
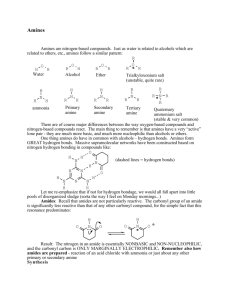
AMINES, AMIDES and ANILINE Amines • An amine is a base as well as a nucleophile • Some amines are heterocyclic compounds (or heterocycles) • Most drugs, vitamins, and many other natural products are heterocycles • A natural product is a compound synthesized by a plant or an animal Organic bases are amines Amines are derivatives of ammonia H N H R N H H H A m m o n ia P rim a ry (1 ) A m in e R N o R H N R R R o S e c o n d a ry (2 ) A m in e o T e rtia ry (3 ) A m in e N 1s2, 2s2 2p1 2p1 2p1----------- lone pair occupies an sp3 orbital Amines are bases because of the lone pair on the nitrogen atom - red litmus paper to blue H H Cl NH 2 N H Cl H B ase + A c id = A m m o n iu m S a lt O H O O O H O o x a lic a c id + 2 N( C H 2 C H 3 ) 3 trie th yla m in e O O + 2 H N( C H 2 C H 3 ) 3 O trie th yla m in iu m o x a la te The lone-pair electrons on nitrogen allows an amine to turn “inside out” rapidly at room temperature Some Common Amines 1,4-butanediamine NH 2 H 2N P u tre s c in e (fo u n d in d e c a yin g m e a t) NH 2 Both upper amines are 1o A m p h e ta m in e (d a n g e ro u s s tim u la n t) N N Is o p ro p yla m in e H T rie th yla m in e P ip e rid in e This amine is are 2o This amine is 3o NH 2 This amine is 1o Aniline can be converted into useful diazonium salt N H2 N a N O 2, H C l N N + C l- 0 C b e n z e n e d ia z o n iu m c h lo rid e N N + Cl - Nuc - - N N Nuc Relative Reactivity of Amine most reactive least reactive RCH2F > RCH2OH ~ RCH2OCH3 > RCH2NH2 HF H2O RCH2OH NH3 pKa = 3.2 pKa = 15.7 pKa = 15.5 pKa = 36 The leaving group of a protonated amine cannot dissociate to form a carbocation or be replaced by a halide ion Reactions of Amines nucleophilic substitution reactions CH 3 C H 2 Br CH 3 N H 2 + CH 3 C H 2 NH CH 3 CH 3 C H 2 NH 2 CH 3 + HBr Br nucleophilic acyl substitution reactions O O C CH 3 C H 2 + Cl 2 C CH 3 N H 2 CH 3 C H 2 + NH C H 3 CH 3 N H 3 Cl Oxidation of Amines oxd R oxd NH 2 R NH oxd OH R O O- R R R NH + H 2O 2 N+ R H + OH - R R N OH R R R + H 2O 2 R + H 2O N OH R N+ R a nitroso compound a hydroxylamine R N O N+ OH R + OH - R N O_ + H 2O R Nitrite Ion, Nitrous Acid, and Nitrosyl Cation – •• •• O •• •• O •• N •• H + •• O H •• + H • O •• • H O •• N H H •• •• + •• N + + •• O •• O •• H •• N •• O •• Example CH3NH2+HNO2 •• (CH3)2NH CH3OH+N2+H2O NaNO2, HCl H2O •• (CH3)2N •• N •• O •• (88-90%) Nitrosation of Secondary Alkylamines + N •• •• O •• N •• N H H O •• N + H N •• •• •• + •• N + •• O •• + nitrosation of secondary amines gives an Nnitroso amine Nitrosation of Primary Arylamines gives aryl diazonium ions aryl diazonium ions are much more stable than alkyl diazonium ions most aryl diazonium ions are stable under the conditions of their formation (0-10°C) + RN + ArN N N fast + R + N2 slow + Ar + N2 Example: (CH3)2CH NH2 NaNO2, H2SO4 H2O, 0-5°C (CH3)2CH + N N HSO4– Transformations of Aryl Diazonium Salts Ar Ar Cl Ar CN + N Ar Ar Ar F Ar I N H Ar Br OH Azo Coupling Diazonium salts are weak electrophiles. React with strongly activated aromatic compounds by electrophilic aromatic substitution. Ar + N N + Ar' H Ar N N Ar' an azo compound Ar' must bear a strongly electron-releasing group such as OH, OR, or NR2. Example OH + + C6H5N N OH N NC6H5 Cl– Amides R' ------------- Not acids or bases R' N C R O N C R O Features of a Peptide Bond 1. 2. 3. 4. Usually inert Planar to allow delocalisation Restricted Rotation about the amide bond Rotation of Groups (R and R’) attached to the amide bond is relatively free