Amines: Structure, Classification, Properties & Basicity
advertisement

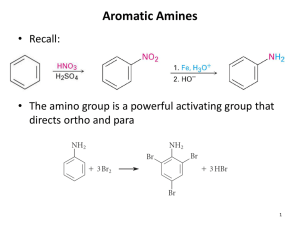
Structure and Classification Amines are classified as 1°, 2°, or 3° depending on the number of carbon groups bonded to nitrogen. Aliphatic amine: All carbons bonded to nitrogen are derived from alkyl groups. See the three above. Aromatic amine: One or more of the groups bonded to nitrogen are aryl groups. Structure and Classification • Heterocyclic amine: An amine in which the nitrogen atom is part of a ring. • Heterocyclic aliphatic amine: A heterocyclic amine in which the ring is saturated (has no C=C bonds). • Heterocyclic aromatic amine: The amine nitrogen is part of an aromatic ring. Nomenclature IUPAC names • We derive IUPAC names for aliphatic amines just as we did for alcohols. • Drop the final -e of the parent alkane and replace it by -amine. • Use a number to locate the amino group on the parent chain. Nomenclature Common names • For most aliphatic amines, list the groups bonded to nitrogen in alphabetical order in one word ending in the suffix -amine. Nomenclature Amine salts • When four atoms or groups of atoms are bonded to a nitrogen atom, as for example CH3NH3+, nitrogen bears a positive charge and is associated with an anion as a salt. • Name the compound as a salt of the corresponding amine. • Replace the ending –amine (or aniline or pyridine or the like) by -ammonium (or anilinium or pyridinium or the like) and add the name of the anion. Physical Properties Like ammonia, low-molecular-weight amines have very sharp, penetrating odors. • Trimethylamine, for example, is the pungent principle in the smell of rotting fish. • Two other particularly pungent amines are 1,4butanediamine (putrescine) and 1,5-pentanediamine (cadaverine). Physical Properties Figure 16.1 Amines are polar compounds: • Both 1° and 2° amines have N-H bonds, and can form hydrogen bonds with one another. • 3° Amines have no N-H bond and cannot form hydrogen bonds with one another. Physical Properties • An N-H---N hydrogen bond is weaker than an O-H---O hydrogen bond, because the difference in electronegativity between N and H (3.0 - 2.1 = 0.9) is less than that between O and H (3.5 - 2.1 = 1.4). • We see the effect of hydrogen bonding between molecules of comparable molecular weight by comparing the boiling points of ethane, methanamine, and methanol. Physical Properties • All classes of amines form hydrogen bonds with water and are more soluble in water than are hydrocarbons of comparable molecular weight. • Most low-molecular-weight amines are completely soluble in water. • Higher-molecular-weight amines are only moderately soluble in water or are insoluble. Basicity of Amines Like ammonia, amines are weak bases, and aqueous solutions of amines are basic. • The acid-base reaction between an amine and water involves transfer of a proton from water to the amine. Basicity of Amines • Aliphatic amines have about the same base strength, and are slightly stronger bases than NH3. • Aromatic and heterocyclic aromatic amines are considerably weaker bases than aliphatic amines. • Note that while aliphatic amines are weak bases by comparison with inorganic bases such as NaOH, they are strong bases among organic compounds. Basicity of Amines • Given the basicities of amines, we can determine which form of an amine exists in body fluids, say blood. • In a normal, healthy person, the pH of blood is approximately 7.40, which is slightly basic. • If an aliphatic amine is dissolved in blood, it is present predominantly as its protonated (conjugated acid) form. Basicity of Amines • Assume that the amine, RNH2, has a pKb of 3.50 and that it is dissolved in blood, pH 7.40 (pOH 6.60). • We first write the base dissociation constant for the amine and then solve for the ratio of RNH3+ to RNH2. • Substituting values for Kb and OH- gives: Reactions of Amines The most important chemical property of amines is their basicity. • Amines, whether soluble or insoluble in water, react quantitatively with strong acids to form water-soluble salts. Basicity of Amines Example: Complete each acid-base reaction and name the salt formed. Basicity of Amines Example: Complete each acid-base reaction and name the salt formed. Solution: