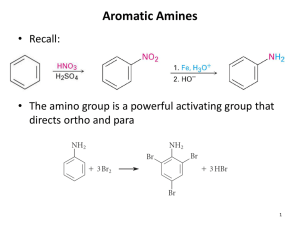
Chem 3.5 #6 Amines
4.
5.
3.
2.
CHEMISTRY 3.5 Name:
1.
WORKSHEET SIX ORGANIC CHEMISTRY Amines
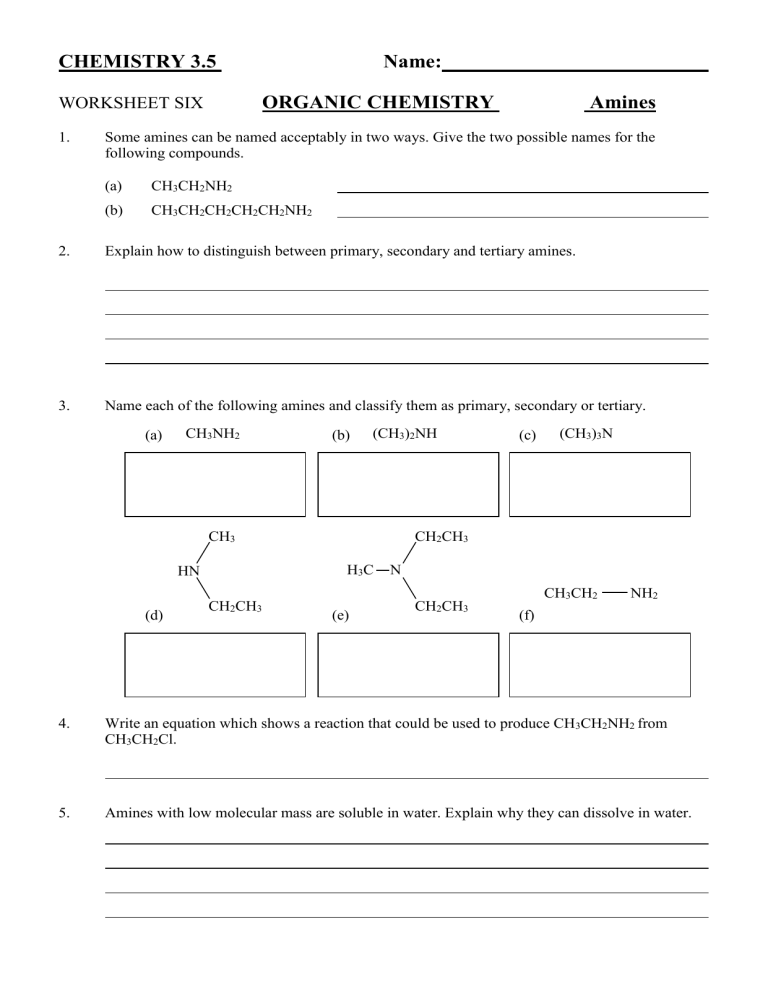
Some amines can be named acceptably in two ways. Give the two possible names for the following compounds.
(a) CH
3
CH
2
NH
2
(b) CH
3
CH
2
CH
2
CH
2
CH
2
NH
2
Explain how to distinguish between primary, secondary and tertiary amines.
Name each of the following amines and classify them as primary, secondary or tertiary.
(a)
(d)
CH
3
NH
2
HN
CH
3
CH
2
CH
3
(b)
(e)
(CH
3
)
2
NH
H
3
C N
CH
2
CH
3
CH
2
CH
3
(c)
(f)
(CH
3
)
3
N
CH
3
CH
2
NH
2
Write an equation which shows a reaction that could be used to produce CH
3
CH
2
NH
2 from
CH
3
CH
2
Cl.
Amines with low molecular mass are soluble in water. Explain why they can dissolve in water.
6.
7.
9.
8.
Primary amines have a lower boiling point than the corresponding alcohols. Explain why.
Amines can be thought off as derivatives of ammonia. Describe the relative strengths of primary, secondary, and tertiary amines as bases, compared to ammonia.
When amines dissolve in water they act like the weak base ammonia, and accept a proton from the water molecule. Complete the following equations to show this reaction occurring.
(a)
(b)
(b)
CH
(CH
3
3
CH
CH
2
2
NH
)
2
2
+ H
2
NH + H
O
2
O
(c) (CH
3
CH
2
)
3
N + H
2
O
Amines can also react directly with acids to form salts. Eg ethylammonium chloride.
(a) Write the equation of the reaction of the ethylammonium ion with water.
Comment on the pH of the solution produced in (a)
10. Complete the equations for the following acid-base reactions.
(a) CH
3
CH
2
NH
2
+ HCl →
(b) (CH
3
CH
2
)
2
NH + H
2
SO
4
→
(c) (CH
3
CH
2
)
3
N + HNO
3
→
(d) CH
3
NH
2
+ H
2
SO
4
→
11. Write names for the products formed above in Question 10.
(a)
(b)
(c)
(d)