Amines

ORGANIC CHEMISTRY
CHM 207
CHAPTER 9:
AMINES
NOR AKMALAZURA JANI
• Amines:
- organic derivatives of ammonia with one or more alkyl or aryl groups bonded to the nitrogen atom.
• Functional group:
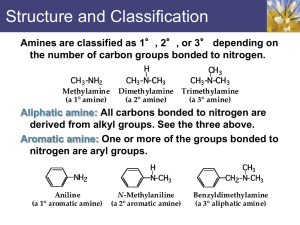
• Classification of amines:
Primary amine Secondary amine Tertiary amine
• Primary (1 o ) amine : one alkyl or aryl group attached to the nitrogen atom.
• Secondary (2 o ) amine : two alkyl or aryl group attached to the nitrogen atom.
• Tertiary (3 o ) amine : three alkyl or aryl group attached to the nitrogen atom.
• Quaternary (4 o ) amine : an ion in which nitrogen is bonded to four alkyl or aryl groups and bears a positive charge
H
3
C
CH
3
N
CH
3
CH
3
NAMING AMINES
• Common names:
- formed from the names of the alkyl groups bonded to nitrogen, followed by the suffix –amine .
- the prefixes di-, tri-, and tetra- are used to decribe two, three or four identical substituents.
CH
3
CH
2
NH ethyl amine
2
CH
3
N
CH
3 cyclohexyldimethyl amine
CH
3
CH
2
CH
3
N
CH
3 ethyldimethyl amine
(CH
3
CH
2
CH
2
CH
2
)
4
N
+ -
CI tetrabutyl ammonium chloride
• IUPAC names:
- similar to that alcohols.
- the longest continuous chain of carbon atoms determine the root name.
- the –e in alkane name is changed to –amine , and a number shows the position of the amino group along the chain.
- other substituents on the carbon chain are given numbers, and the prefix Nis used for each substituent on nitrogen.
CH
3
4
CH
3
2
NH
2
CH CH
3
2 1
2-butanamine
CH
3
4
CH
3
CH
3
CH
2
2
NH
2
CH
1
2
3-methyl-1-butanamine
CH
3
4
3
CH
2
NHCH
3
CH CH
2
1
3
N-methyl-2-butanamine
CH
3
CH
3
CH
3
CH
2
CH CH CH CH
3
N CH
3
CH
3
2,4, N, N-tetramethyl-3-hexanamine
• The prefix ‘amino’ is used to indicate the presence of an –NH
2 group in a molecule containing than one functional group.
• For example,
NH
2
CH
2
COOH aminoethanoic acid
OH
NH
2
H
2
NCH
2
CH
2
OH
2-aminoethanol
COH
NH
2
2,4-diaminophenol
NH
2
3-aminobenzaldehyde
NAMING AROMATIC PRIMARY AMINES
• Aromatic amines have an amine group (-NH
2 to the aromatic ring.
) attached directly
• Aromatic amines known as arylamines.
• Examples,
NH
2 phenylamine
(aniline)
3
CH
3
2
NH
2
1
4
6
5
2-methylphenylamine
(2-methylaniline)
NH
2
1
2
6
5
4
NO
2
3
4-nitrophenylamine
(4-nitroaniline)
• Compounds with two –NH
2 groups are named by adding the suffix ‘diamine’ to the name of the corresponding alkane or aromatic compounds.
H
2
N (CH
2
)
6
NH
2 hexane-1,6-diamine
(1,6-hexanediamine)
H
2
N NH
2 benzene-1,4-diamine
(1,4-benzenediamine)
PHYSICAL PROPERTIES OF AMINES
i) Boiling points:
- the boiling points of amines is increase with increasing relative molecular mass.
- the lower aliphatic amines are gases or low-boiling liquids.
- amines are polar compounds and both primary and secondary amines associate by intermolecular hydrogen bonding.
H
H N
R H
R
N
H
Hydrogen bonding
H
R
N
H
* Comparing the boiling points of 1 o , 2 o and 3 o amines
- for isomeric amines, the boiling points decreases in the order,
1 ° amine > 2 ° amine > 3 ° amine
- reason: decrease in intermolecular hydrogen bonding.
- example,
CH
3
CH
2
CH
2
NH
2
1-propanamine
(1 o
amine) boiling point: 48.6
o
C
CH
3
CH
3
CH
2
N H
N-methylethanamine
(2 o
amine)
37.0
o
C molecular formula: C
3
H
9
N molecular mass: 59
CH
3
CH
3
N CH
3
N, N-dimethylmethanamine
(3 o
amine)
3.5
o
C
* Comparing the boiling points of amines with other organic compounds
- the boiling points of aliphatic amines are higher than those of alkanes or haloalkanes of similar relative molecular mass due to intermolecular hydrogen bonding.
- the N-H bond is more polar than the C-H bond but less polar than O-H bond. Hydrogen bonding in amines are weaker than that of alcohols or carboxylic acids. Boiling points of amines are lower than those corresponding alcohols or carboxylic acids.
Comparison of boiling points of some organic compounds with similar molecular weight alkane < ether < alkyl halide < amine < ketone, aldehyde < alcohol < acid
ii) Solubilities of 1 o , 2 o and 3 o amines:
- all three classes of aliphatic amines are capable of forming hydrogen bonds with water molecules.
- the lower amines (with chain length up to four carbon atoms per molecule) are very soluble in water because they can form hydrogen bonds with water molecules.
- the solubilities of amines is decrease with increasing number of carbon atoms in the chain.
- amines are soluble in organic solvents.
THE BASICITY OF AMINES
• Amines can act as:
- a nucleophile (a Lewis base) because its lone pair none bonding electrons can form a bond with an electrophile.
- a Brønsted-Lowry base because it can accept a proton from a proton acid.
Reaction of an amine as a nucleophile
H
H
R N CH
3
I
H nucleophile electrophile
R N CH
3
I
-
H new N-C bond formed
Reaction of an amine as a proton base
R N base
H
H
H X proton acid
H
R N H
H protonated
X
-
• Amines are fairly strong base and their aqueous solutions are basic.
• An amine can abstract a proton from water, giving an ammonium ion and a hydroxide ion.
• The equilibrium constant for this reaction is called base-dissociation constant, symbolized by K b
.
R N
H
H
H O H
K b
R
H
N H OH
-
H
K b
= [RNH
3
+ ] [
-
OH]
[RNH
2
] pK b
= - log
10
Stronger base have smaller values of pK b
K b
• The basicity of the amines depends on the ability of the lone pair none bonding electrons at nitrogen atom to form bond with an acid.
• The more easier the lone pair electrons formed bond with the acid, will make the amines a stronger base.
• Factors that effect the basicity of the amines: i) substitution by alkyl groups
- the presence of alkyl groups (electron-donating group) such as (CH
3
) and (CH
3
CH
2
-) will make the amine become more basic.
-
- for example, methylamine is more basic than ammonia.
ii) substitution by electron-withdrawing groups
- the presence of electron-withdrawing groups or atom will decrease the basicity.
- for example, nitroaniline is less basic than aniline
Basicity of aromatic amines
* Aromatic amines is less basic than aliphatic amines and ammonia.
* Reason:
- the lone pair of electrons on the nitrogen atom is delocalised into the benzene ring.
- As a result, the lone pair of electrons is less available for donation to an acid.
- The reaction is shifted toward the left and makes aniline a weaker base than ammonia or aliphatic amines.
REACTIONS OF AMINES
• Salt formation
• Reaction with nitrous acid
• Amide formation
• Ring halogenation of phenylamine
Salt formation
• Reaction of amines and acid will give amine salt.
• Amine salt:
- composed of two types of ions: i) the protonated amine cation (an ammonium ion) ii) anion derived from the acid
• Amine salts are ionic, have higher melting points, nonvolatile solids, more soluble in water than the parent amines and slightly soluble in nonpolar organic solvents.
R NH
2 primary amine
HCl
R
2
NH HCl secondary amine
R
3
N tertiary amine
EXAMPLES:
HCl
CH
3
CH
2
CH
2
NH
2 n-propylamine
HCl
(CH
3
CH
2
)
3 triethylamine
N HCl
RNH
3
Cl alkylammonium chloride
R
2
NH
2
Cl dialkylammonium chloride
R
3
NHCl trialkylammonium chloride
CH
3
CH
2
CH
2
N H
3
Cl n-propylammonium chloride
(CH
3
CH
2
)
3
N H Cl triethylammonium chloride
Reaction with nitrous acid
• Nitrous acid (HNO
2
) is unstable and is prepared in situ by the reaction of dilute HCl or dilute H
2
SO
4 with sodium nitrite in the absence of heat.
NaNO
2
(s) + HCl (aq) → NaCl (aq) + O=N-OH (aq) nitrous acid
• Nitrous acid can be used to differentiate primary, secondary and tertiary aliphatic amines.
Primary aliphatic amines
• When aliphatic primary amines react with HNO
2
, nitrogen is evolved rapidly and an alcohol is produced.
RNH
2
+ O= N -OH → R-OH + H
2
O + N
2
(g)
• For example, ethylamine gives nitrogen and a mixture of ethanol
(60%), ethene and other products.
C
2
H
5
N H
2
+ O= N -OH → C
2
H
5
-OH + H
2
O + N
2
(g) + other products
• The reaction of propylamine with HNO
2 produces nitrogen and a mixture of 1-propanol (7%), 2-propanol (32%) and propene (28%).
• The reaction of methylamine with HNO
2 produces only a little methanol, and the main products are methoxymethane and nitrogen .
Secondary aliphatic and aromatic amines
• Aliphatic secondary amines react with HNO
2 at room temperature to form nitrosoamines / nitrosamines (yellow oils).
R
2
N-H + HO-N=O → R
2
N-N=O + H
2
O nitrosoamine
• Example,
CH
3
CH
3
N H dimethylamine
HO N=O
CH
3
N N=O H
2
O
CH
3
N-nitroso-N,N-dimethylamine
Tertiary aliphatic amines
• A tertiary aliphatic amines react with HNO
2 will produced ammonium salts which is dissolve readily in water as a clear solution.
R
3
N + HNO
2
→ [R
3
NH] + NO
2
(aq)
Primary aromatic amines
• A primary aromatic amines react with cold HNO
2 and dissolved in dilute HCl at 0-5 o C will produced diazonium salt . When this cold salts heated at room temperature, nitrogen gas will evolved.
NH
2
HNO
2
HCl
5 o
C
N
2
+
Cl
-
2H
2
O
RT
N
2 mixture products benzenediazonium chloride
RT = room temperature
Tertiary aromatic amines
• Tertiary aromatic amines reacts with nitrous acid by undergoing substitution at the para position of the benzene ring to form nitrosoaniline which is a yellow precipitate.
R
N R
HNO
2
< 5 o
C
ON
R
N R a nitosoamiline compound (yellow precipitate)
Amide formation
i) Reaction with acyl chlorides
• Primary and secondary amines are acylated at room temperature by acyl chlorides to form N-substituted amides.
RN
R
2
N
H
H
2
+ CH
3
CO Cl → RNHCOCH
3
+ CH
3
CO Cl → R
2
NCOCH
3
+ HCl
+ HCl
• Tertiary amines are NOT acylated because they do not have hydrogen atom attached to the nitrogen atom.
Examples:
H
CH
3
N H
O
CH
3
C Cl ethanoyl chloride
H
N H
O
CH
3
C Cl ethanoyl chloride
H O
CH
3
N C CH
3
N-methylethanamide
H
N
O
C CH
3
HCl
HCl
N-phenylbenzamide
ii) Reaction with acid anhydrides
• Primary and secondary amines are readily acylated by acid anhydrides to yield the corresponding N-alkyl or N-aryl amides.
• For example,
H
CH
3
CH
2
CH
2
N propylamine
H
H
CH
3
CH
2
N CH
2
CH diethylamine
3
O O
CH
3
C O C CH
3 ethanoic anhydride
O O
CH
3
C O C CH
3 ethanoic anhydride
H O
CH
3
CH
2
CH
2
N C CH
3
N-propylethanamide
O
O
HO C CH
3
CH
3
CH
2
N C CH
3
CH
2
CH
3
N, N-diethylethanamide
O
HO C CH
3
Ring halogenation of phenylamine
• When bromine water is added to phenylamine (aniline) at room temperature, decolorisation of the bromine water occurs and a white precipitate of 2,4-6-tribromoaniline
(C
6
H
4
Br
3
N) is obtained.
• This reaction is used as a test for aniline.
NH
2
3Br
2
(aq) room temperature
Br
NH
2
Br
Br
2,4,6-tribromoaniline
(white precipitate)
3HBr
USES OF AMINES
SYNTHESIS OF NYLON
• Nylons are condensation copolymers formed by reacting equal parts of a diamine and a dicarboxylic acids , so that peptide bonds form at both ends of each monomer in a process analogous to polypeptides biopolymers.
• General reactions:
Dicarboxylic acids Diamines
Nylon
Basic concepts of nylon production
• The first approach:
combining molecules with an acid (COOH) group on each end are reacted with two chemicals that contain amine (NH
2
) groups on each end.
Form nylon 6,6, made of hexamethylene diamine with six carbon atoms and acidipic acid, as well as six carbon atoms.
•
The second approach:
a compound has an acid at one end and an amine at the other and is polymerized to form a chain with repeating units of (-NH-[CH
2
]n-CO-)x.
Form nylon 6, made from a single six-carbon substance called caprolactam.
SYNTHESIS OF DYE
• Primary aromatic amines are used as a starting material for the manufacture of azo dyes.
• Azo compounds:
- compounds bearing the functional group R-N=N-R', in which
R and R' can be either aryl or alkyl.
- N=N group is called an azo group
- HNNH is called diimide
• Aryl azo compounds have vivid colors, especially reds, oranges, and yellows
Yellow azo dye
• Amines react with nitric(III) acid to form diazonium salt, which can undergo coupling reaction to form azo compound.
• Azo-compounds are highly coloured, they are widely used in dyeing industries, such as: i) Methyl orange ii) Direct brown 138 iii)Sunset yellow FCF iv)Ponceau
Uses and important of azo dye
• Methyl orange - used as acid-base indicators due to the different colors of their acid and salt forms
• Artist’s paints – clays, yellow to red range
• Dye in food and textiles
E102: Tartrazine
E107 : Yellow 2G
E110 : Sunset Yellow
E122 : Azorubine
EXAMPLES OF
AZO DYES USED
IN FOOD
E123 : Amaranth
E124 : Ponceau 4R
E129 : Allura Red
E151 : Brilliant Black