Chapter 17 Aromaticity
advertisement

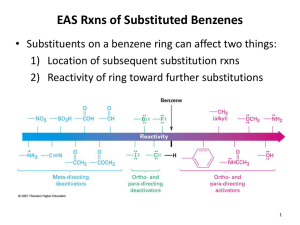
8-methyl-N-vanillyl-6-nonenamide (Capsaicin) Chapter 17: Benzene and Aromaticity Buckminsterfullerene TNT Key points & objectives: • Aromatic molecules are cyclic, conjugated, flat, and unusually stable • 4n + 2 electrons (n = 0, 1, 2, …. • Hydrocarbon aromatics: benzene, naphthalene, anthracene, toluene, xylylene • Heterocyclics: pyridine, pyrimidine, imidazole, pyrrole, thiophene, furan, indole…. • Molecular orbitals using Frost diagrams (inscribed circles) • Ring current deshields NMR signals – downfield Benzene 1st isolated by Michael Faraday in 1825 From “Benzoin,” corrupt form of the Arabic "luban jawi” for the “frankincense of Java” Frankincense Not “aromatic” in the technical sense triterpene Cancer drug anti-inflammatory hepatotoxicity Boswellia sacra Aromatic-fragrant myrth Commiphora myrrha tree Antiseptic, embalming agent, incense Cinnamon (2E)-3-phenylprop-2-enal cinnamaldehyde Diabetes Antimicrobial antioxidant Capsaicin 8-methyl-N-vanillyl-6-nonenamide (Capsaicin) 16,000,000 Scovilles psoriasis relieve the pain of peripheral neuropathy trigger apoptosis in human colon and lung cancer Vanilla Tincture (ethanol extract) of vanilla aphrodisiac and a remedy for fevers catecholamines (including adrenaline) addictive Aromatic molecules • • • • • Flat Conjugated (4n +2) pi electrons Unusually stable Ring current (deshielding protons) Anesthetics & analgesics Advil, and Motrin Sunscreens Only complete UVA block Skin Damage • Very high energy radiation (UVC) is currently blocked by the ozone layer (ozone hole issue) • High energy radiation (UVB) does the most immediate damage (sunburns) • But lower energy radiation (UVA) can penetrate deeper into the skin, leading to long term damage Source: N.A. Shaath. The Chemistry of Sunscreens. In: Lowe NJ, Shaath NA, Pathak MA, editors. Sunscreens, development, evaluation, and regulatory aspects. New York: Marcel Dekker; 1997. p. 263-283. 12 Sources and Names of Aromatic Hydrocarbons From high temperature distillation of coal tar Heating petroleum at high temperature and pressure over a catalyst Aromatics are less reactive than Alkenes Aromatics Nomeclature Aromatics Nomeclature Agent orange Polychlorinatedbiphenyls PCB’s Thermally stable, electrically insulating heat transfer liquid Casting wax for lost wax process for making metal things dichlorodiphenyltrichloroethane Malaria mosquito Mueller 1948 Nobel Prize in Medicine Thermodynamic stability of benzene: Heats of Hydrogenation Monosubstituted Benzenes • Most monosubstituted aromatics are named using -benzene as the parent name preceded by the substituent name (as a prefix; all one word): fluoro nitro ethyl F NO2 CH2CH3 fluorobenzene nitrobenzene ethylbenzene Alkyl-substituted Benzenes • Alkyl substituted benzenes are named according to the length of the carbon chain of the alkyl group. • With six carbons or fewer in the alkyl chain, they are named as ‘alkylbenzene.’ • e.g., propylbenzene: CH2CH2CH3 Alkyl-substituted Benzenes • With more than six carbons in the alkyl chain, they are named as a ‘phenylalkane,’ where the benzene ring is named as a substituent (phenyl) on the alkane chain • e.g., 4-phenylnonane CH 3CH 2CH 2 CH2CH2CH3 CHCH2CH2CH2CH2CH3 = CHCH 2CH 2CH 2CH 2CH 3 CH3CH2CH2CHCH2CH2CH2CH2CH3 4-phenylnonane The Benzyl Group • The benzyl group is a common name for a methyl substituted benzene (toluene) having substitution for one of the hydrogens on the methyl group. CH2 the benzyl group CH2Br benzyl bromide CH2CH OH 2Br benzyl alcohol Common Names of Subs. Benzenes • There are a number of nonsystematic (common) names commonly used for certain monosubstituted benzenes (see next slide) • These ten common names should be memorized. • These common names are used as base names when naming more their more highly substituted derivatives. Examples of these will be given later. Mono-substituted Benzene Nomenclature: Common Names Disubstituted Benzenes • Disubstituted benzenes can be named in one of two ways. Each method describes the relative positions of the two groups on the benzene ring. • Systematic numbering of the aromatic ring. • Using the prefixes ortho-, meta-, or para-. • When numbering the ring carbons, carbon # 1 is always a substituted carbon. • The substituents are listed alphabetically. Disubstituted Benzenes ortho- (abbreviated o- ) = 1,2-disubstituted (two groups on adjacent carbons on the ring) F F CH2CH3 Br o-difluorobenzene or 1,2-difluorobenzene o-bromoethylbenzene or 1-bromo-2-ethylbenzene Disubstituted Benzenes meta- (abbreviated m- ) = 1,3-disubstituted (two groups having one unsubstituted carbon between them) Br Br m-dibromobenzene or 1,3-dibromobenzene Br NO2 m-bromonitrobenzene or 1-bromo-3-nitrobenzene Disubstituted Benzenes para- (abbreviated p- ) = 1,4-disubstituted (two groups on opposite sides of the ring) Br Cl Cl p-dichlorobenzene or 1,4-dichlorobenzene Cl p-bromochlorobenzene or 1-bromo-4-chlorobenzene Disubstituted Benzenes • When one of the substituents changes the base name, either o-, m-, and p- or numbers may be used to indicate the position of the other substituent. • Carbon # 1 is always the carbon bearing the substituent that changes the base name. Br 4 1 OH 3 1 2 Cl 2 NH 2 p-bromoaniline or 4-bromoaniline o-chlorophenol or 2-chlorophenol Common Names of Disubs. Benzenes • There are a few nonsystematic (common) names for disubstituted benzenes that you should be familiar with: CH3 CH3 CH3 CH3 CH3 o-xylene m-xylene CH3 CH3 p-xylene OH o-cresol CH3 CH3 OH m-cresol OH p-cresol Disubstituted Benzenes Relative positions on a benzene ring ortho- (o) on adjacent carbons (1,2) meta- (m) separated by one carbon (1,3) para- (p) separated by two carbons (1,4) Describes reaction patterns (“occurs at the para position”) Polysubstituted Benzenes • Polysubstituted benzenes must be named by numbering the position of each substituent on the ring (with more than two substituents, o-, m-, and p-can NOT be used.) • The numbering is carried out to give the substituents the lowest possible numbers. Carbon #1 always has a substituent. • List the substituents alphabetically with their appropriate #s. CH2CH3 F 1 2 3 4 NO2 2-ethyl-1-fluoro-4-nitrobenzene Polysubstituted Aromatics having a Common base name • Common names of the monosubstituted benzenes are used as parent names for polysubstituted aromatics when one of the substituents changes the base name. • For such rings with common names, the carbon bearing the substituent responsible for the common name is always carbon #1. • The substitutents are toluene CH3 listed in alphabetical 1 Cl chloro order. bromo Br 5 2 3 4 5-bromo-2-chlorotoluene Polysubstituted Benzenes Br 4 OH 1 3 2 CH2CH3 1 NO2 4-bromo-2-ethyl-1-nitrobenzene Cl 2 Br 5 3 4 5-bromo-2-chlorophenol Polysubstituted Benzenes Br 2 3 O2N 4 Br 1 1 CH3 6 6 Cl 5 2-bromo-6-chloro-4-nitrotoluene O2N 5 4 2 CH2CH3 3 Cl 1-bromo-3-chloro-2-ethyl-5-nitrobenzene Naming Benzene as a Substituent • A benzene substituent is called a phenyl group, and it can be abbreviated in a structure as “Ph-”. • Therefore, benzene can be represented as PhH, and phenol would be PhOH. 38 Polycyclic Aromatic Hydrocarbons (PAH) anthracene naphthalene phenanthrene pyrene benzo [a] pyrene Metabolic byproducts of benzo [a] pyrene react with DNA to form adducts, leading to carcinogenesis (cancer). Naphthalene Orbitals Three resonance forms and delocalized electrons 40 13C NMR Absorptions of Dibromobenzenes Figure 17.2 • The number of signals (lines) in the 13C NMR spectrum of a disubstituted benzene with two identical groups indicates whether they are ortho, meta, or para to each other. 41 Drugs that Contain a Benzene Ring Figure 17.5 42 Heterocyclic Aromatics Heterocyclic Aromatics Pyridine A six-membered heterocycle with a nitrogen atom in its ring electron structure resembles benzene (6 electrons) The nitrogen lone pair electrons are not part of the aromatic system (perpendicular orbital) Pyridine is a relatively weak base compared to normal amines but protonation does not affect aromaticity 45 Protonation of Pyrroles and Pyridines Pyrrole A five-membered heterocycle with one nitrogen electron system similar to that of cyclopentadienyl anion Four sp2-hybridized carbons with 4 p orbitals perpendicular to the ring and 4 p electrons Nitrogen atom is sp2-hybridized, and lone pair of electrons occupies a p orbital (6 electrons) Since lone pair electrons are in the aromatic ring, protonation destroys aromaticity, making pyrrole a very weak base 47 Structure and Stability of Benzene: Molecular Orbital Theory Benzene reacts slowly with Br2 to give bromobenzene (where Br replaces H) This is substitution rather than the rapid addition reaction common to compounds with C=C, suggesting that in benzene there is a higher barrier Heats of Hydrogenation as Indicators of Stability The addition of H2 to C=C normally gives off about 118 kJ/mol – 3 double bonds would give off 356kJ/mol Two conjugated double bonds in cyclohexadiene add 2 H2 to give off 230 kJ/mol Benzene has 3 unsaturation sites but gives off only 206 kJ/mol on reacting with 3 H2 molecules Therefore it has about 150 kJ more “stability” than an isolated set of three double bonds 50 32 kcal/mole Benzene’s Unusual Structure All its C-C bonds are the same length: 139 pm — between single (154 pm) and double (134 pm) bonds Electron density in all six C-C bonds is identical Structure is planar, hexagonal C–C–C bond angles 120° Each C is sp2 and has a p orbital perpendicular to the plane of the six-membered ring 52 The Criteria for Aromaticity Four structural criteria must be satisfied for a compound to be aromatic: 1. A molecule must be cyclic. • To be aromatic, each p orbital must overlap with p orbitals on adjacent atoms. 53 The Criteria for Aromaticity 2. A molecule must be planar. • All adjacent p orbitals must be aligned so that the electron density can be delocalized. • Since cyclooctatetraene is nonplanar and not aromatic, it undergoes addition reactions just like those of other alkenes. 54 The Criteria for Aromaticity 3. A molecule must be completely conjugated. • Aromatic compounds must have a p orbital on every atom. 55 The Criteria for Aromaticity 4. A molecule must satisfy Hückel’s rule, and contain a particular number of electrons. Hückel's rule: • Benzene is aromatic and especially stable because it contains 6 electrons. • Cyclobutadiene is antiaromatic and especially unstable because it contains 4 electrons. 56 • Hückel’s rule refers to the number of electrons, not the number of atoms in a particular ring. Why 4n +2? When electrons fill the various molecular orbitals, it takes two electrons (one pair) to fill the lowest-lying orbital and four electrons (two pairs) to fill each of n succeeding energy levels This is a total of 4n + 2 58 Bonding and Antibonding Orbitals • The combination of two p orbitals can be constructive—that is, with like phases interacting—or destructive, that is, with opposite phases interacting. • When two p orbitals of similar phase overlap side-by-side, a bonding molecular orbital results. • When two p orbitals of opposite phase overlap side-by-side, a * antibonding orbital results. 59 Formation of π and π* Molecular Orbitals • Two atomic p orbitals combine to form two molecular orbitals. • The bonding MO is lower in energy than the two p orbitals. • The * antibonding MO is higher in energy because a destabilizing node results, which pushes nuclei apart when orbitals of opposite phase combine. Figure 17.8 60 Molecular Orbitals for Benzene • Since each of the six carbon atoms in benzene has a p orbital, six atomic p orbitals combine to form six MOs. Figure 17.9 61 Inscribed Polygon Method of Predicting Aromaticity 62 Inscribed Polygon Method of Predicting Aromaticity • This method works for all monocyclic completely conjugated systems regardless of ring size. • The total number of MOs always equals the number of vertices of the polygon. • The inscribed polygon method is consistent with Hückel's 4n + 2 rule—there is always one lowest energy bonding MO that can hold two electrons and the other bonding MOs come in degenerate pairs that can hold a total of four electrons. 63 Inscribed Polygon Method of Predicting Aromaticity Figure 17.10 64 Buckminsterfullerene—Is it Aromatic? • Buckminsterfullerene (C60) is a third elemental form of carbon. • Buckminsterfullerene is completely conjugated, but it is not aromatic since it is not planar (CAREFULL!!!) • It undergoes addition reactions with electrophiles in much the same way as ordinary alkenes. 65 Compounds With 4n Electrons Are Not Aromatic (May be Antiaromatic) Planar, cyclic molecules with 4 n electrons are much less stable than expected (antiaromatic) They will distort out of plane and behave like ordinary alkenes 4- and 8-electron compounds are not delocalized (single and double bonds) Cyclobutadiene is so unstable that it dimerizes by a self-Diels-Alder reaction at low temperature Cyclooctatetraene has four double bonds, reacting with Br2, KMnO4, and HCl as if it were four alkenes 66 Aromatic Ions The 4n + 2 rule applies to ions as well as neutral species Both the cyclopentadienyl anion and the cycloheptatrienyl cation are aromatic The key feature of both is that they contain 6 electrons in a ring of continuous p orbitals 67 Aromaticity of the Cyclopentadienyl Anion 1,3-Cyclopentadiene contains conjugated double bonds joined by a CH2 that blocks delocalization Removal of H+ at the CH2 produces a cyclic 6-electron system, which is stable Removal of H- or H• generates nonaromatic 4 and 5 electron systems Relatively acidic (pKa = 16) because the anion is stable 68 Cycloheptatriene Cycloheptatriene has 3 conjugated double bonds joined by a CH2 Removal of “H-” leaves the cation The cation has 6 electrons and is aromatic 69 NMR and Aromaticity • 1H NMR spectroscopy readily indicates whether a compound is aromatic. • The protons on sp2 hybridized carbons in aromatic hydrocarbons are highly deshielded and absorb at 6.5–8 ppm, whereas hydrocarbons that are not aromatic absorb at 4.5–6 ppm. 70 Larger Aromatic Rings • Completely conjugated rings larger than benzene are also aromatic if they are planar and have 4n + 2 electrons. • Hydrocarbons containing a single ring with alternating double and single bonds are called annulenes. • To name an annulene, indicate the number of atoms in the ring in brackets and add the word annulene. 71 Hückel’s Rule and Number of Electrons • [10]-Annulene has 10 electrons, which satisfies Hückel's rule, but a planar molecule would place the two H atoms inside the ring too close to each other. • Thus, the ring puckers to relieve this strain. • Since [10]-annulene is not planar, the 10 electrons cannot delocalize over the entire ring and it is not aromatic. 72 Biochemically Relevant Aromatics Amino Acids Biologically Relevant Aromatics Nicotinamide adeine dinucleotide, the biolgical hydrogenator NADH NAD+