Lecture 2
advertisement

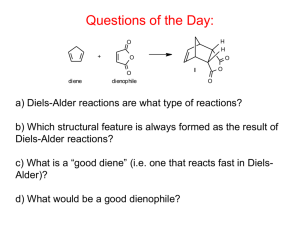
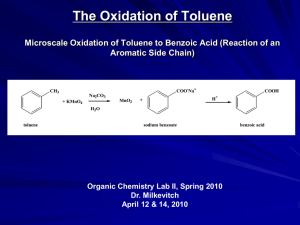
Outline for Today Review MO construction for Conjugated Systems Discuss Diels-Alder Reaction Chapter 14 – Aromaticity Tie in Aromaticity to Diels-Alder Further Reading: Structure and Mechanism in Organic Chemistry By Felix A. Carrol Chapter 4.1-2 – MO theory/aromaticity Chapter 11.4 Cycloadditions Lectures available online at http://perceco2.chem.upenn.edu/~percec/classes.html Until further notice. MO’s for Ethene MO’s for Allyl Systems MO’s for Butadiene MO’s for Hexatriene Diels-Alder Reaction: The Basics S-cis diene required, s-trans does not work Concerted reaction Bond made and broken simultaneously A Simple Example: 2+2 Cycloadditons Out of phase In Phase Suprafacial vs. Antarafacial Cycloaddition Molecular Orbitals of Diels Alder Cycloaddition Normal Electron Demand/ Thermal Diels-Alder Inverse-Electron Demand/ Photochemical Diels-Alder Normal versus Inverse Electron Demand Normal Inverse Woodward Hoffman Rules for Cycloadditons “A Reaction occurs when the bonding electrons of a product can be transferred, without a symmetry imposed barrier, to the bonding orbitals of the product.” Last Chapter of “The Conservation of Orbital Symmetry” – Exceptions? “There are none.” Overall Diels Alder Transition State Stereochemistry of Diels-Alder Reactions: Effect of Dienophile Structure Stereochemistry of Diels-Alder Reactions: Effect of Diene Structure Diels Alder Approaches: Regio and Stereochemistry Endo-Selectivity: Secondary Orbital Overlap Controlling Regioselectivity of Diels Alder Reactions Interacting Orbitals Asymmetrically Amplified to create regioselectivit Other types of Diels-Alder Reactions Lecture 2: Aromatic Compounds Chapter 14 in Solomons 9/e History of the Benzene Structure Example of Aromatic Compounds: Motivation Diels-Transition State Fullerenes Brief Note on Benzene Nomenclature Aromatic Stabilization: Resonance Stabilization Benzene Immune to Many Standard De-aromitizing Reactions Note: There is an error in the diagram on page 605 of Solomons. MO Description of Benzene E=α+2β E=α+β E=α-β E=α-2β Overall stabilization=8β, compared to 6β for 3 ethenes or 7β for hexatriene Hückel’s Rule/Frost Circles 4n+2 π – electrons = high stabilization due to ideal filling of bonding orbitals More stable than linear polyene equivalents, closed shell configuration 4n π electron = anti-aromatic 1,3-cyclobutadiene Less stable than linear butadiene – open shell configuration Does not exist under non stabilized conditions Aromatic and Nonaromatic Annulenes: Application of 4n and 4n+2 rules Polycyclic Aromatics Benzene NMR Aromatic Ring Currents 1H NMR for aromatic Hydrogens δ: 6.0-9.5 ppm 13C NMR for aromatic Carbon δ:100-170 ppm The Allotropes of Carbons b,d,e,f,h all aromatic Cylcopentadienyl Cations and Anions Anti Aromatic Aromatic Heterocyclic Aromatics Heterocyclic Aromatics Protonation of Pyrolles and Pyridines Biochemically Relevant Aromatics Amino Acids Biologically Relevant Aromatics Nicotinamide adeine dinucleotide, the biolgical hydrogenator NADH NAD+ Diels-Alder and Hückel Theory Transition state has 6=4n+2 where n=1 electrons, therefore is aromatic and low in energy, despite high entropic cost. Note: If Diels-Alder Substrate is Aromatic to begin with will often not participate.