File - Mrs. Dawson`s Classroom
advertisement

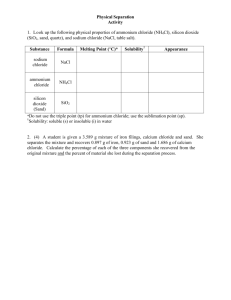
Name: Period: The Color of Fireworks Step 1: Make an observation The normal electron configuration of atoms or ions of an element is known as the “ground state”. In this most stable energy state, all electrons are in the lowest energy levels available. When atoms in their ground state are heated to high temperatures, some electrons gain enough energy to move to higher energy levels. The element is then said to be in the “excited state”. This excited configuration is unstable and the electrons fall back to their normal positions of lower energy. While releasing the energy, they emit the energy in the form of electromagnetic energy. Some of this energy is in the visible spectrum of light. This is what produces the colorful flames in fireworks. Today, you will observe the characteristic colors produced by certain metallic ions in aqueous using a flame test. Step 2: Ask Questions 1. What characteristic colors will be produced by various metallic ions? 2. What test will we use to determine the metal ions? Step 3: Create a Hypothesis If energy in the form of a flame is used to test metallic ions, then________________________________ _____________________________________________________________________________________ Step 4: Conduct an Experiment 1. What safety precautions should we take? 2. What will our independent variable be? 3. What will our dependent variable be? 4. What will be our control group? 5. How will we clean the spatula to avoid contamination? Materials: 7 small beakers filled (and label) with the following solutions: o 15ml Sodium chloride o 15ml Potassium chloride o 15ml Lithium chloride o 15ml Calcium chloride o 15ml Strontium chloride o 15ml Copper (II) chloride o 15ml Barium chloride 1 small beaker filled with 30mL of HCl Expo marker- to label beakers Bunsen Burner Rubber tubing Spatula Safety goggles Procedure: 1. 2. 3. 4. 5. 6. 7. 8. 9. Gather and check all materials and lab equipment. Place 7 beakers in a row in on your lab bench away from flame. Dip spatula into one of the solutions, completely immersing the tip. Hot spatula over flame and observe the color change. Turn down the lights if needed. Record data. a. All flames will start out blue in color or invisible but will change to the colors characteristic of the metal salts. b. The copper chloride will begin to burn after a few minutes. Be sure thoroughly extinguish this fire with water. Dip the spatula into the HCl solution. Repeat steps 3-6 for the remaining 6 solutions. Clean up lab station, turn off burner and see Ms. Stine for discard instructions. Have lab station checked by TA or Ms. Stine Step 5: Record Data Chloride Solution Color of Flame Sodium chloride Potassium chloride Lithium chloride Calcium chloride Strontium chloride Copper (II) chloride Barium chloride Step 6: Conclusion: 1. How does this experiment relate to Chemistry? 2. When an element or compound is placed in a burning solution, the atoms absorb energy and promote electrons to “excited” energy levels, which are different from their normal ground state. Explain how this creates colored light. 3. What is the name of this spectrum of specific wavelengths produced by exciting an element? 4. Why is the spectrum different for every element? 5. Write the formula for each compound used in today’s lab.