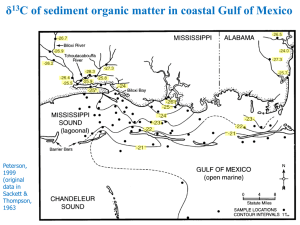
δ - Indiana University
advertisement
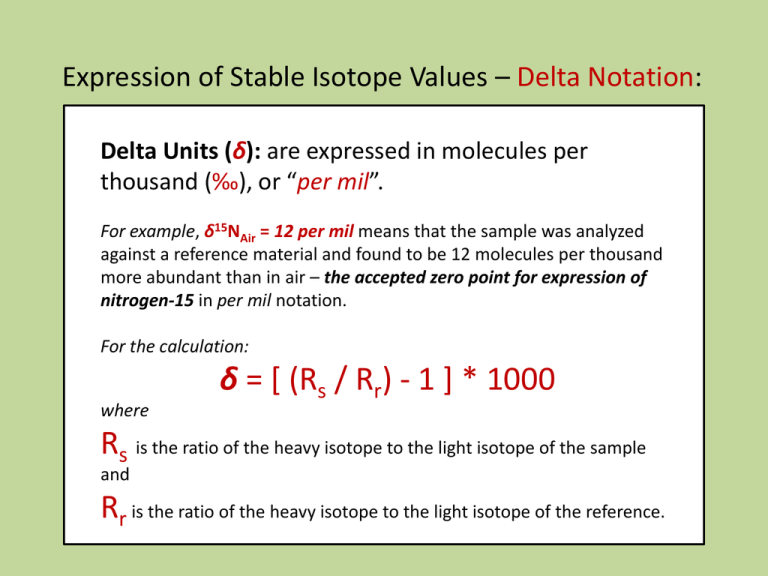
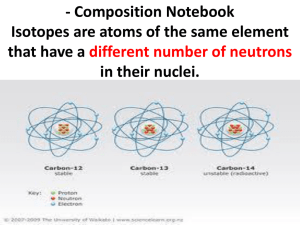
Expression of Stable Isotope Values – Delta Notation: Delta Units (δ): are expressed in molecules per thousand (‰), or “per mil”. For example, δ15NAir = 12 per mil means that the sample was analyzed against a reference material and found to be 12 molecules per thousand more abundant than in air – the accepted zero point for expression of nitrogen-15 in per mil notation. For the calculation: where δ = [ (Rs / Rr) - 1 ] * 1000 Rs is the ratio of the heavy isotope to the light isotope of the sample and Rr is the ratio of the heavy isotope to the light isotope of the reference. Isotope Ratio Mass Spectrometer – “IRMS” bending magnet A mass spectrometer is an instrument which separates charged molecules by mass. An isotope ratio mass spectrometer (IRMS) works on this principle, but unlike other conventional mass spectrometers it has been specifically designed to measure the proportions of particular isotopes. An IRMS will be much more precise, but much less sensitive than other mass spectrometers. Ionisation Principle of a Quadrupole Mass Spectrometer For mass separation, quadrupole systems use a high-frequency alternating electrical field. A Faraday cup and a secondary electron multiplier serve as detectors. Commonly used in conjunction with either gas-chromatography or liquid-chromatography, and more recently with ICP, as a simple high throughput screening system. A quadrupole has four parallel rods that have fixed DC and alternating RF potentials applied to them. Ions produced in the source of the instrument are then focused and passed along the middle of the quadrupoles. Stable Isotopes – Mass Dependant Fractionation Rayleigh Effects two species (or phases as in water) that are in equilibrium with one another, or where the reactants and products become separated from one another Kinetic Effects kinetic effects are most commonly seen in processes that are influenced by biologic processes, during unidirectional processes Diffusion the light isotope of an element will diffuse more rapidly than the heavy isotope The most widely studied stable isotopes are: Element Isotopes Hydrogen 1H, 2H 10B, 11B Boron 12C, 13C Carbon Nitrogen 14N, 15N 16O, 17O, 18O Oxygen 32S, 33S, 34S, 36S Sulfur α is the isotope fractionation factor, (e.g. α for water liquid and vapor is 10‰ i.e., the δ18O of the liquid is 10‰ heavier). α is typically calculated in K (degrees Kelvin) which is equal to oC + 273. α is the isotope fractionation factor, (e.g. α for water liquid and vapor is 10 ‰, i.e., the δ18O of the liquid is 10‰ heavier). The size of this isotopic fractionation can be expressed in several ways: Natural log notation, 1000ln(α): 1000ln(α) – 1000 ln (1- ε) = ε ε The epsilon notation, : εA-B = (αA-B – 1) * 1000 The epsilon notation has the advantage over the 1000ln(α) notation in that it is an exact expression of the per mil fractionation. The capital delta notation, Δ: Δ A-B = δA - δB Some General “Rules” • Isotope fractionation factors are greater at lower temperatures. • Light isotopes are enriched in biogenic compounds. • Light isotopes are enriched in reduced species and heavy isotopes are enriched in oxidized species (e.g., the δ13C of CO2 is higher than that of CH4). • It follows from this that reactions which involve a change in oxidation state result in a greater degree of stable isotope fractionation than those that do not. • Where two minerals of the same oxidation state are in equilibrium with one another, the mineral with the heaviest cation will have the lightest stable isotope composition (e.g., the δ 34S of ZnS (sphalerite) is higher than that of PbS (galena)). • The extent of stable isotope fractionation is inversely proportion to the square of the relative mass difference between two isotopes. This means that the extent of stable isotope fractionation between 100Ru and 101Ru is less than 1% of that between 10B and 11B. In practice this has meant that stable isotope fractionation is effectively below detection limits for elements with masses greater than 40 (i.e., for elements with masses greater than that of Ca). Recent advances in mass spectrometry are increasing the range of elements for which stable isotope variations can be detected. Ranges of O Isotopic Values in Geologic Systems meteoric waters ocean water – VSMOW = 0 ‰ sedimentary rocks metamorphic rocks granitic rocks basaltic rocks extraterrestrial materials (meteorites and lunar rocks) -70 -60 -50 -40 -30 -20 -10 0 10 20 30 40 δ18O (‰ VSMOW) Oxygen isotopes of meteoric water are generally lighter than those of seawater (Hoefs, 1980, fig. 10). Rainout effect on δ2H and δ18O values; (based on Hoefs 1997 and Coplen et al. 2000). Change in the 18O content of rainfall according to a Rayleigh distillation, starting with δ18Ovapor = -11‰, temp. = 25°C, and final temp. of -30°C. Note that at 0°C, fractionation between snow and water vapor replaces rainvapor fractionation. The fraction remaining has been calculated from the decrease in moisture carrying capacity of air at lower temperatures, starting at 25°C. Dashed lines link δ18O of precipitation with temperature of condensation. (Reproduced from Clark and Fritz 1997, p.48) W Ocean Water (Clark and Fritz 1997, p. 37, as compiled in Rozanski et al. 1993, modified by permission of American Geophysical Union). http://www.sahra.arizona.edu/programs/isotopes/images/diagram7.gif Vienna Standard Mean Ocean Water (VSMOW) is a water standard defining the isotopic composition of water. It was promulgated by the International Atomic Energy Agency in 1968. Despite the misleading designation ocean water, VSMOW does not include any salt or other substances usually found in seawater and refers to pure water with a particular composition of isotopes. VSMOW serves as a reference standard for comparing hydrogen and oxygen isotope ratios, mostly in water samples. Very pure, distilled VSMOW water is also used for making high accuracy measurement of water's physical properties and for defining laboratory standards since it is considered to be representative of average ocean water, in effect representing all water on Earth. 0‰ 0‰ http://www.sahra.arizona.edu/programs/isotopes/images/diagram7.gif The isotopic composition of VSMOW water is specified as ratios of the molar abundance of the rare isotope in question divided by that of its most common isotope and is expressed as parts per million (ppm). For instance 16O (the most common isotope of oxygen with eight protons and eight neutrons) is roughly 2,632 times more prevalent in sea water than is 17O (with an additional neutron). The isotopic ratios of VSMOW water are defined as follows: 2H/1H = 155.76 ±0.1 ppm (a ratio of 1 part per approximately 6420 parts) 3H/1H = 1.85 ±0.36 × 10−11 ppm (a ratio of 1 part per approximately 5.41 × 1016 parts, ignored for physical properties-related work) 18O/16O = 2,005.20 ±0.43 ppm (a ratio of 1 part per approximately 498.7 parts) 17O/16O = 379.9 ±1.6 ppm (a ratio of 1 part per approximately 2,632 parts) Annual Climate Records: Variation in the closely spaced rings of a bristlecone pine correspond to annual changes in rainfall and temperature. (Photograph copyright Henri D. Grissino-Mayer) sclectarian corals An ice core with a layer of algae. Water vapor gradually loses 18O as it travels from the equator to the poles. Because water molecules with heavy 18O isotopes in them condense more easily than normal water molecules, air becomes progressively depleted in 18O as it travels to high latitudes and becomes colder and drier. In turn, the snow that forms most glacial ice is also depleted in 18O. As glacial ice melts, it returns 16O-rich fresh water to the ocean. Therefore, oxygen isotopes preserved in ocean sediments provide evidence for past ice ages and records of salinity. (Illustration by Robert Simmon, NASA GSFC based on data provided by Cole et. al. 2000, archived at the World Data Center for Paleoclimatology). http://earthobservatory.nasa.gov/Features/Paleoclimatology_Evidence/ A Greenland ice core with a layer of algae. global-warming.accuweather.com/ice_core_algae From Gercke, in preparation Cyprideis sp. Perissocytheridea sp. Age y.b.p. From Gercke, in preparation δ18O Large scale Climate Records: Diagram showing the nature of the loess stratigraphic record. In most regions, including much of North America, Europe, and China, loess was deposited during glacial periods and soils were formed during interglacial periods. Soils that become buried by younger loess are called "paleosols." http://esp.cr.usgs.gov/info/eolian/task2.html Global Records: http://earthobservatory.nasa.gov/Features/Paleoclimatology_Evidence/ Shriner, C.M., Elswick, E.R., Ripley, E.M., Shimmelmann, A., and Murray, H.H., (in press) Natural Environment as a Determinative Factor in Greek Early Helladic Cultural Change on the Argive Plain: in Katsonopoulou, D. Ed., The Early Helladic Peloponnesos, Helike IV; The Heike Society; Athens, Greece. Shriner, C.M., Elswick, E.R., Ripley, E.M., Shimmelmann, A., and Murray, H.H., (in press) Natural Environment as a Determinative Factor in Greek Early Helladic Cultural Change on the Argive Plain: in Katsonopoulou, D. Ed., The Early Helladic Peloponnesos, Helike IV; The Heike Society; Athens, Greece. Ranges of C Isotopic Values in Geologic Systems freshwater ΣCO2 shallow ocean ΣCO2 deep ocean ΣCO2 marine organic C land plants soil organic C volcanic CO2 biogenic methane soil CO2 sedimentary organic matter, petroleum, coal marine and non-marine organisms freshwater carbonates marine carbonates atmospheric CO2 carbonatites, diamonds extraterrestrial materials (meteorites and lunar rocks) -50 -40 -30 -20 -10 0 10 20 30 40 50 60 δ13C (‰ VPDB) Carbon isotopes in geologic systems. Carbonate carbon derived from seawater is much heavier than organic carbon, and hence than carbonate formed by oxidation of organic matter (Hoefs, 1980, fig. 9; Trumbore and Druffel, 1995). Diagrammatic Representation of the Exogenic Cycles of Carbon and Sulfur Carbonate Carbon 6460 x Car 1018 moles δ13C = -0.4 Gypsum 166 x 1018 moles δ34S= +8.4 SW sulfate 40 x 1018 moles δ34S= +20 Ocean Carbonate, DIC 3.3 x 1018 moles δ13C = +0.46 Pyrite Organic Carbon 180 x 1018 moles δ34S= -7.9 1180 x 1018 moles δ13C = -27 S - Cycle C - Cycle Gregor et al.. 1988 The denominations are because in the plants of group C3, the first photosynthesized organic compound has 3 atoms of carbon while in group C4, there are 4. (There is also a third, very minor, group called CAM, a combination of C3 and C4 where some cactus and succulents belong to.) http://homepage.mac.com/uriarte/carbon13.html Most plants (85%) (e.g. trees and crops) follow the C3 photosynthesis pathway and have lower values of δ13C, between -22‰ and -30‰. The remaining 15% of the plants are of type C4. The majority are tropical herbs and have high values of δ13C, between –10 ‰ and –14 ‰. Carbon-13 ---------------------C3 and C4 plants Almost 99% of atmospheric CO2, contains the less heavy carbon, 12C. A small part, 1.1% of CO2, is somewhat heavier, since it contains 13C. Terrestrial vegetation and marine phytoplankton, in the process of photosynthetic absorption of CO2, discriminate against heavy molecules preferring 12C to 13C. In this way, the carbon trapped in continental flora contains a smaller proportion of 13C than the carbon in atmospheric CO2. CAM C3 C4 frequency The isotopes of a chemical element are the various configurations of its atoms. There are three carbon isotopes in nature: 12C, 13C and 14C. These are three varieties of the same chemical element, carbon, whose nuclei contain the same number of protons (six), but a different number of neutrons (six, seven and eight, respectively). Thus, besides having the same chemical properties, the isotopes have different atomic masses: twelve, thirteen and fourteen, respectively. -30 -20 -10 δ13C (‰ VPDB) C3 – plants The isotopic signature of C3 plants shows higher degree of 13C depletion than the C4 plants. Examples of C3 plants include wheat, rice, soybeans, maples. An illustration of the stomatal CO2 proxy. (Left) Photomicrograph of fossil leaf cuticle of the fern aff. Stenochlaena from just after the Cretaceous/Tertiary (K/T) boundary. (Right) The fern's nearest living relative, Stenochlaena palustris. The stomatal index of the fossil cuticle is considerably lower than the extant cuticle, indicating that CO2 was higher directly after the K/T boundary than today (21). Photos courtesy of Barry Lomax (University of Sheffield, Sheffield, U.K.). (Scale bars, 10 μm.) www.pnas.org/content/105/2/407/F2.large.jpg C4 – plants Over 8000 species of angiosperms have developed adaptations which minimize the losses to photorespiration developed in the Oligocene (25-32 mya) and became ecologically important in the Miocene (5-12 mya). The formula for calculating δ13C (in ‰) is as follows: (13C/12C)sampled – (13C/12C)standard ——————————––––––––––––––– x 1.000 (13C/12C)standard These C4 plants are well adapted to (and likely to be found in) habitats with high daytime temperatures and intense sunlight, as well as potential nitrogen or CO2 limitations. Some examples: crabgrass, corn (maize), sugarcane , sorghum Although only ~3% of the angiosperms, C4 plants are responsible for ~25% of all the photosynthesis on land. Increase in δ13C from palaeosols and tooth enamel showing apparent synchronicity in the transition to C4dominated terrestrial ecosystems across continents. Data for (a) from Cerling et al. (1997), (b) from Quade & Cerling (1995) and (c) from Passey et al. (2002). wetter dryer www.enviroone.com/images/crops/corn.jpg Present-day C4 plants are concentrated in the tropics (below latitudes of 45°) where the high air temperature contributes to higher possible levels of oxygenase activity by rubisco. www.illinoistimes.com Monocot examples: Forty-six percent of grasses are C4 and together account for 61% of C4 species. Dicot examples: Members of the sedge family Cyperaceae, and numerous families of Eudicots, including the daisies Asteraceae, cabbages Brassicaceae, and spurges Euphorbiaceae also use C4. Vienna- PeeDee Belemnite standard -- V-PDB The common reference for delta 13C Marine Carbonate Standard was obtained from a Cretaceous marine fossil, Belemnitella americana, from the PeeDee Formation in South Carolina. This material has a higher 13C/12C ratio than nearly all other natural carbon-based substances. For convenience it is assigned a delta 13C value of zero, giving almost all other naturally-occurring samples a positive delta value. The original sample was used up long ago, but the IAEA calibrated a new reference sample to the original fossil, giving rise to the widespread use of the term ViennaPeeDee Belemnite standard, abbreviated to V-PDB. www.ucmp.berkeley.edu/.../belemnite_anatomy.gif ucmp.berkeley.edu www.nhm.ac.uk/.../fossil_types/belemnites.htm Bullet-shaped fossils called belemnites occur commonly in rocks of Jurassic and Cretaceous age (65-205 million years old). They can be found weathered out of clays and chalks in great abundance at some localities. These examples are from the Pee Dee Formation, Florence County, South Carolina. Pee Dee Formation, Florence County, SC www.blackriverfossils.org Ranges of S Isotopic Values in Geologic Systems evaporate sulfate ocean water sedimentary rocks metamorphic rocks granitic rocks basaltic rocks extraterrestrial materials (meteorites and lunar rocks) -50 -40 -30 -20 -10 0 10 20 30 40 50 60 δ34S (‰ VCDT) Range of sulfur isotopes in geologic systems. Note the difference between mantle-derived S and Sedimentary sulfides (Hoefs, 1980, fig. 12). Diagrammatic Representation of the Exogenic Cycles of Carbon and Sulfur Carbonate Carbon 6460 x Car 1018 moles δ13C = -0.4 Gypsum 166 x 1018 moles δ34S= +8.4 SW sulfate 40 x 1018 moles δ34S= +20 Ocean Carbonate, DIC 3.3 x 1018 moles δ13C = +0.46 Pyrite Organic Carbon 180 x 1018 moles δ34S= -7.9 1180 x 1018 moles δ13C = -27 S - Cycle C - Cycle Gregor et al.. 1988 weathering 72 104 MOR volcanic emissions 10 sea-salt sulfur 144 deposition over oceans 207 some as OCS to stratosphere volatile biological emissions 22 volatile biological emissions 43 some from explosive volcanism to stratosphere deposition over continents 32 volcanic emissions 10 eolian erosion 10 Natural Sulfur Cycle precipitation of evaporites BSR to pyrite http://www.libraryindex.com/article_images/www.libraryindex.com/sulfur.01.jpg&imgrefurl=http://www.libraryindex.com/pages/3406/Sulfur- Depth (m) GENERALIZED STRATIGRAPHY (YAX-1) AND SAMPLE DISTRIBUTION (n=46) rst.gsfc.nasa.gov/Sect18/Chicxulub d34S sulfates (‰) -30 -20 -10 0 10 20 30 40 700 Paleocene 800 900 Cretaceous 1100 W.S.S. A.S.S. 1200 1300 R.S. Cretaceous Seawater Sulfate (Kampschulte and Strauss, 2004) Depth (m) 1000 1400 (Keller et al., 2004) 1500 Evolution of the sulfide and sulfate reservoirs. BSR – bacterial sulfate reduction www.lifesci.dundee.ac.uk Evolution of the sulfide and sulfate reservoirs Biomineral formation by fungi and sulphate-reducing bacteria. (A) a cord-forming fungus growing on copper phosphate (B) light micrograph of moolooite crystals (copper oxalate, CuC2O4.xH2O) around the hyphal cords (C) scanning electron micrograph of moolooite crystals associated with hyphal cord and mucilaginous sheath (Fomina, M. et al. (2005) Applied and Environmental Microbiology 71, 371-381) (D) a crust of calcium oxalate (weddelite and whewellite) crystals and tubular crystalline sheath around fungal hyphae (ESEM dry mode) (Gadd, G.M. et al. (2006) In: Fungi in the Environment, Cambridge University Press. (E) hydrated sulphate-reducing bacterial biofilm (Desulphomicrobium sp.) transforming selenite to abundant Se/S granules. Inset shows granules associated with the surface of an individual bacterium and precipitation in the extracellular matrix (Hockin, S.L. & Gadd, G.M. (2003) Applied and Environmental Microbiology 69, 7063-7072). Vienna- Canyon Diablo Triolite -- V-CDT The Canyon Diablo meteorite comprises many fragments of the asteroid that impacted at Barringer Crater (Meteor Crater), Arizona, about 50,000 years ago. Meteorites have been found around the crater rim, and are named for nearby Canyon Diablo, which lies about three to four miles west of the crater. Pyrrhotite var.Triolite: An unusual iron sulfide mineral in which the ratio of iron to sulfur atoms is somewhat variable Fe(1-x)S (x = 0 to 0.2) but is always slightly less than 1. It commonly is found in association with other sulfides. The variety troilite, with a composition near that of iron sulfide (FeS), is an important constituent of some iron-nickel meteorites. Canyon Diablo Troilite (CDT) is used as a zero standard of relative concentration of different isotopes of sulfur. A meteoritic standard was chosen because of the constancy of the sulfur isotopic ratio in meteorites, while the sulfur isotopic composition in Earth materials varies owing to bacterial activity. VCDT is the synthetic standard -0.3‰ more depleted than the original CDT.