Introduction to Environmental Isotopes
advertisement

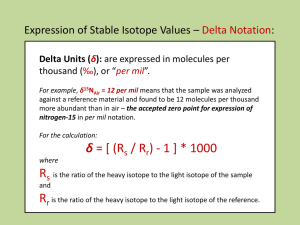
Nucleosynthesis of the nuclides Chart 2nd Generation Stars (Our Sun) Fusion by CNO reaction + 1H 13N + g 13N 13C + b+ + v 13C + 1H 14N + g 14N + 1H 15O + g 15O 15N + b+ + v 15N + 1H 12C + 4He 12C 12C + 4 1H 12C + 3 g + 2 b+ + 2 v Then by combustion of 16O ( < 1 yr), a reaction that produces higher mass elements, including Si and Mg 16O + 16O 28Si + 4He + g 12C + 16O 24Mg + 4He + g chart Fusion is limited at mass 56Fe by the diminishing return of energy of fusion and increased energy of nuclear bonds p = proton = 1.007593 u = 1.6726234 E–27 kg n = neutron = 1.008982 u = 1.6749287 E–27 kg u = 1 atomic mass unit = 1/12 12C = 1.660018 E–27 kg 56 26Fe30 = 26p A 56Fe = 56 + 30n Mais le poid atomique de 56Fe = 55.934942 (http://csnwww.in2p3.fr/AMDC/web/masseval.html) 26 x 1.007593 = 26.197418 u 30 x 1.008982 = 30.269460 u 56.466878 u 56.466878 – 55.934942 = 0.531936 u = 0.883 E–27 kg = masse perdue Converti en énergie de liason nucléaire: E = mc2 Energy of nuclear bonding • maximum at 56Fe • beyond, fusion is an endothermic reaction • nucleosynthesis beyond 56Fe by neutron capture reactions and by fission of the nuclides with Z > 90 (uranium and the transuranics) http://www.chem.uidaho.edu/~honors/nucbind.html End as a red giant and a supernova Supernova remnants Cas A in x-rays (Chandra) Cygnus Loop (HST): green=H, red=S+, blue=O++ Vela Remnant of SN386, with central pulsar (Chandra) SN1998bu Nucleosynthsis in 2nd generation stars: Inventory – 1H, 4He, 12C, 13C, 14N, 15N, 16O, 20Ne, 24Mg, 28Si, 32S, 36Ar, 40Ca, 44Ca,48Ti, 52Cr, 56Fe Production of neutrons: 13C + 4He 16O + n Nucleosynthesis by neutron and proton capture Process S – slow neutron capture (2nd generation stars) Production of elements up to Bi Process R – capture of fast neutrons (red giants) Production of heavy elements – to U. After is limited by fission Process P – proton capture (1H) Production of nuclides poor in neutrons s 50 Very unstable nuclides (T½ < 1 day) Unstable nuclides (T½ > 1 day) 40 Atomic Number (Z) m Z=N Stable nuclides 30 20 Isotopes 10 Isobars Isotones 0 0 10 20 30 Neutrons (N) 40 50 The Stable Environmental Isotopes Isotope Ratio 2H 3He 13C 15N 18O 34S 37Cl % natural abundance 2H/1H 0.015 3He/4He 0.000138 13C/12C 1.11 15N/14N 0.366 18O/16O 0.204 34S/32S 4.21 37Cl/35Cl 24.23 Reference VSMOW Atmospheric He VPDB AIR N2 VSMOW, VPDB CDT SMOC Routine and non-routine stable environmental isotopes Isotope Ratio D 2 13 13 1.11 11,100 15 15 0.366 3,660 AIR N2 (3.677·10–3) 18 18 0.204 2,040 34 34 4.21 42,100 VSMOW (2.0052 · 10–3) VPDB (2.0672 · 10–3) CDT (4.5005 · 10–2) 3 He 3 He/4He 0.000138 6 Li 6 Li/7Li 7.5 75,000 C N O S H/1H C/12C N/14N O/16O S/32S 0.015 150 Reference (abundance ratio) Commonly analysed compounds VSMOW (1.5575 · 10–4) H2O in water and ice, CH4 and other hydrocarbons, mineral hydration waters, DIC, DOC, carbonate minerals, organics, CO2, CH4 and other hydrocarbons, N2 in groundwater, NO3–, NH4+, N2O, organic compounds H2O, dissolved O2, SO42–, NO3–, Carbonate minerals (VPDB) SO42–, H2S, sulfate and sulfide minerals VPDB (1.1237 · 10–2) 1.38 AIR (0.00000138) Groundwater, minerals L-SVEC (0.08362) Seawater, brines, groundwater, minerals, hydrothermal water Seawater, carbonates, groundwaters, bines, Cl– and chloride minerals, chlorinated hydrocarbons, perchlorate compounds Seawater, brines, groundwaters, carbonates, Ca-minerals and rocks. 11 11 80.1 801,000 NBS 951 (4.044) 37 37 24.23 242,300 SMOC (0.324) 87 87 B Cl Sr B/10B natural abundance % ppm Cl/35Cl Sr/86Sr 7.0 and 9.8 Direct measurement Delta - permil: d - ‰ m ( O / O ) sam ple m ( O / O ) reference 18 d 18 O sam ple d 18 O sam ple 16 18 18 16 16 m ( O / O ) reference ( 18 O / 16 O ) sam ple 18 1 1000 16 ( O / O ) reference ‰ VSMOW What is the relative enrichment or depletion of crustal rocks (~0.204%) relative to VSMOW d 18 18O O R crustal 0.00204 16 O crustal 18 O R V SM O W 0.0020052 16 O V SM O W 18 O crustal R crustal 0.00204 3 3 1 10 1 10 0.0020052 R V SM O W = 17.4‰ VSMOW crustal rocks are enriched in 18O by 17.4‰ or 1.7% relative to the standard VSMOW in Isotope Ratio Mass Spectrometry Laser attenuation isotope analyser (Wavelength-Scanned Cavity Ring Down Spectroscopy – WS-CRDS) • Laser absorption • Reads fraction of heavy isotope bonds • Direct reading of BOTH • Do it in the field! 18O and D ratios Laser attenuation isotope analyser (Wavelength-Scanned Cavity Ring Down Spectroscopy – WS-CRDS) and Picarro – nice Los Gatos – the original black box small footprint Laser attenuation isotope analyser (Wavelength-Scanned Cavity Ring Down Spectroscopy – WS-CRDS) Check out the sample requirements – 2 mL. Fill a tray of 100! – lots of good data. Distribution of isotopes in nature • Isotope fractionation during reaction • Rayleigh distillation during reservoir depletion Isotope fractionation, a H2Owater « H2Ova po ur R reactant a R product a 18 O water vapor O 16 O water O 16 O vapor 18 18 a 18 O water d 18 O sam ple a 18 vapor O 16 O water O 16 O vapor 18 18 ( 18 O / 16 O ) sam ple 1000 18 1 16 ( O / O ) reference O w ater vapor O 16 O w ater O 16 O vapor 18 18 d d 18 18 O w ater 1000 O vapour 1000 Physico-chemical fractionation 16 K [ 18 [ 16 O water + O ] wat [ 16 O ] wat [ 18 K 18 O vap o r O ] vap O ] vap [ 18 O ] wat [ 16 O ] wat [ 18 O ] vap [ 16 O ] vap [ 18 [ 16 18 O water + O ] wat O ] wat R wat R vap [ 16 [ 18 16 O vap o r O ] vap O ] vap [ 18 O ] wat [ 16 O ] wat [ 18 O ] vap [ 16 O ] vap a wat vap 1 Isotope partitioning functions = symmetry value m = mass of isotope E = the energy state summed from the zero-point to the energy of the dissociated molecule (J·mole–1) k = Boltzmann constant (gas constant per molecule) = n · 1.380658 · 10–23 JK–1 T = thermodynamic temperature K (Q (Q * X * Y ) ) e E / kT Energy absorbed m 3/2 Energy released Q 1 0 zero point of enegy / QX RX = = a XY RY / QY bond w ith heavy isotope Interatomic istance Diffusive fractionation v kT 2 m v = molecular velocity (cm · s–1) k = Boltzmann constant (gas constant per molecule) = n · 1.380658 · 10–23 JK–1 m = molecular mass (e.g. 7.3665 · 10–26 kg for 12C16O2) T = absolute temperature K Diffusive Fractionation e.g. 13C during CO2 diffusion Diffusion in a vacuum a 13 C CO 2 kT v (1 3 CO 2 v (1 2 CO 2) ) 2 45 kT 44 0 . 9888 45 2 44 Diffusion in air a 13 C CO 2 m 12 ( m 13 m air ) m 13 ( m 12 m air ) 44 ( 45 28 . 8 ) 45 ( 44 28 . 8 ) 0 . 9956 Metabolic (biologic) Fractionation • 13C during photosynthesis • sulphate reduction • methanogenesis . . . Units Isotope Enrichment (e) • Isotope difference in permil units between two reacting phases at equilibrium e X Y RX 3 3 1 10 ( a 1) 10 RY • when a is small, then we can use: e X Y 10 ln a X Y 3 Units Isotope Separation (D) • Isotope difference in permil units between any two phases D X Y d X d Y For a water – vapor exchange at 25°C what is the d18O of vapor, where: • water d18Ow = 0.0 ‰ VSMOW For a water – vapor exchange at 25°C what is the d18O of vapor, where: • water d18Ow = 0.0 ‰ VSMOW The fractionation factor (a) is: a18Ow-v = 1.0093 The isotopic enrichment (e): and e18Ow-v = (a–1) ·103 = 9.3‰ e18Ov-w = – 9.3‰ For a water – vapor exchange at 25°C what is the d18O of vapor, where: • water d18Ow = 0.0 ‰ VSMOW e18Ow-v = (a–1) ·103 = 9.3‰ d18Ovapor = d18Owater – e18Owater-vapor = 0.0 – 9.3‰ = – 9.3‰ • vapor d18Ov = –9.30‰ VSMOW For most reactions in hydrogeology: • d values are typically –50 to +50 ‰ • a values are close to 1 (0.98 to 1.02) • e values are typically –20 to +20 ‰ Except for some extreme reactions and light isotopes . . . e.g. hydrogen gas produced from water is strongly depleted in 2H and has a fractionation factor a2HH2O-H2 = 3.76 at 25°C. What will be the d2H value for H2 produced from water with d2HH2O = –75‰ at 25°C? a 2 H H 2O H 2 3.76 R H 2O R H2 d 2 H H 2 O 1000 d H H 2 1000 2 3.76 ( 75 ) 1000 d 2 H H 2 1000 d2HH2 = –754‰ VSMOW (but using e, d2HH2 = –75 – 2760 = –2835‰) So, use the e simplification . . . • when a is close to 1 • when the d-values are not too different from the reference (i.e. within a few tens of permil of 0) Fractionation and Temperature Q (Q (Q 40 30 T °C 20 18 10 O w-v * X * Y 1 m 3/2 ) ) e E / kT / QX RX = = a XY RY / QY lnaX-Y = aT–2 + bT–1 + c 0 18 -10 O i-v -20 8 10 12 14 103 lna18O 16 18 Fractionation and Temperature 103 lna2H 60 80 100 140 160 40 40 30 30 2 20 H w-v 20 18 O w-v 10 10 0 0 2 18 -10 O i-v -20 H i-v T °C T °C 120 -10 -20 8 10 12 14 103 lna18O 16 18 Fractionation and Temperature eD ‰ 60 80 100 140 160 40 40 30 30 2 18 10 O H w-v 20 w-v 10 0 0 2 18 -10 O i-v -20 H i-v T °C 20 T °C 120 -10 -20 8 10 12 14 e 18O ‰ 16 18 T°C 2 H water-vapour AA 1 A 150 25 ?? ?? ?? ?? 13C 10°-40° 73.2 -303.9 -204.34 -8.949 25 -60 -7.69 181.264 346 151 6.1 -90.888 -223 -38 88.4 1 0 0 0 1.137 1.534 5.9702 290.498 2.1 23.9 21.3 -0.4156 -3.206 -32.801 -127.9 -22 -7.9 2.8 -2.0667 2.644 52.227 1.137 -0.0206 2.78 -1.8034 -0.4156 17.9942 0 10.611 1.0 -19.97 -2.89 -2.7798 3.2 0.45 0 3.25 2.88 2.88 3.88 0 0 2.3 0 0 0 0 -1.5 -0.4 -3.7 -5.1 -4.1 -3.6 -2.9 29.1 2.8 4.0 31.5 28.3 28.8 25.0 1.029571 1.002836 1.004022 1.031958 1.0287 1.029215 1.025278 50 50 200 3.52 1.9189 3.55 0 8.582 0 -4.350 -18.977 -2.570 29.4 26.0 13.3 1.029791 Kita et al., 1985 1.026294 Kawabe, 1978 1.013375 Shiro and Sakai, 1972 34°-93° 0°-100° 195°-573° Alkali feldspar-H2O Ca- feldspar-H2O Kaolinite-H2O Smectite-H2O Chlorite-H2O 500 500 200 150 25 3.13 2.09 2.5 2.67 1.56 0 0 0 0 0 -3.7 -3.7 -2.87 -4.82 -4.7 1.5 -0.2 8.3 10.1 12.8 1.001537 0.999796 1.008331 1.010142 1.012931 Bottinga and Javoy, 1973 Bottinga and Javoy, 1973 Land and Dutton, 1978 Yeh and Savin, 1977 Wenner and Taylor, 1971 500°-800° 500°-800° CO2(g)-CO2(aq) HCO3-CO2(g) HCO3-CO2(aq) CO3-HCO3 CO3-CO2(g) CO3-CO2(g) CaCO3-HCO3 CO2-CaCO3 " 25 25 25 25 25 25 25 25 25 25 200 200 0.942 0 0 0 0 0.87 0 0 -2.988 -0.42615 -1.04 -4.2 -8.815 0.373 9.552 9.866 -0.867 0 9.037 -4.232 7.6663 -6.4449 -2.45 12.079 18.393 -0.19 -24.10 -24.12 2.52 -3.40 -22.73 15.10 -2.46 16.30 9.90 -5.95 -8.9 1.1 7.9 9.0 -0.4 6.4 7.6 0.9 -10.4 -10.1 -10.0 0.8 -0.5 1.001062 1.007968 1.00901 0.999612 1.006407 1.007608 1.000907 0.989693 0.989942 0.990034 1.000819 0.999492 Vogel, Grootes and Mook, 1970 Mook, Bommerson and Staverman (1974) Mook, Bommerson and Staverman (1974) Mook, Bommerson and Staverman (1974) Deines et al. (1974) IAEA (1983) Thode et al., 1965 Mook, 1986 Bottinga, (1968) Bottinga, (1969) Bottinga, (1969) Bottinga (1969) Bottinga (1969) 200 0.8914 -8.557 18.11 -8.27 0.2 300 300 -0.62 2.28 6.616 15.176 6.04 -8.38 -3.08 SiO2(amorph)-H2O SiO2(quartz)-H2O SiO2(quartz)-H2O CO2-CaCO3 CO2-CH4 CO2-CH4 34S Temperature Ran 2 2 1.011231 1.078511 #NUM! 1.019487 1.020814 1.020814 1.135457 2.583595 3.485485 3.762008 2.11556 2.419961 1.02008 1.022608 1.027229 2.357616 0.985154 1.177903 1.16855 1.009373 1.009189 1.009416 1.003105 1.002804 1.014859 1.040966 1.031062 1.012601 Majzoub (1971) Kakiuchi and Matsuo, 1979 Northrop and Clayton, 1966 Sheppard and Schwarcz, 1970 Fontes (1965) Lloyd (1968) McKenzie and Truesdell (1977) Mitzutani and Rafter (1969) Lloyd (1968) Fractionation - Other Systems ice-vapour water vapour - hydrogen gas " water-hydrogen gas methane-hydrogen gas 1 " water vapour-methane water vapour-methane Water-methane water-H2S water-gypsum water-horneblend water-biotite water-vapour " " water-ice " ice-vapour CO2-H2O Calcite-H2O CO2-Calcite Aragonite-H2O Dolomite-H2O Dolomite-Calcite water-gypsum SO4-H2O " " anhydrite-H2O 0 0 0 0 100 25 25 100 100 25 25 25 25 25 100 100 25 25 25 0 0 0 25 15 25 25 50 100 25 25 25 25 100 " " 52.612 -168 -161.04 Reference -76.248 467.6 389.61 water-ice -76.248 64.55 794.84 a 24.844 0 13 1158.8 24.844 2.408 -1620.1 103lna C 11 76 10953246 19.3 20.6 20.6 127 949 1249 1325 749 884 20 22 27 858 -15 164 156 9.3 9.1 9.4 3.1 2.8 14.7 40.1 30.6 12.5 " 18O B 6 O'Neil (1968) Arnason (1969) Suzuoki and Kumura (1973) Majzoub (1971) plus Arnason (1969) Suess (1949) 100°-200° Bottinga (1969) calculated 0°-600° Bottinga (1969) plus Majzoub (1971) Horibe and Craig (1975) written communication, in Friedman Bottinga (1969) calculated 0-700 Bottinga (1969a) calculated 0°-700° Bottinga (regressed) 10-250 Bottinga (1969a) plus Majzoub (1971) 0°-100° Galley et al. (1972) 25°-200° Gonfiantini and Fontes (1967) Suzuoki and Epstein (1976) 450°-850° Suzuoki and Epstein (1976) 450°-850° Majzoub (1971) Bottinga and Craig (1969) Kakiuchi and Matsuo, 1979 10°-40° O'Neil (1968) Suzuoki and Kumura (1973) Majzoub (1971) plus O'Neil (1968) Bottinga (1968) 0-100 O'Neil, Clayton and Mayeda (1969) 0°-500° Bottinga, 1968 0°-600° 200°-800° 100°-650° 0°-500° 110°-200° 100°-575° 5°-125° 5°-125° 5°-125° <100° <100° <100° <100° <100° 0-280 200-700 0-700 1.000198 Ohmoto and Rye (1979) <600° 25.0 24.3 1.025354 Bottinga, (1969) 1.024602 Richet et al. (1977) 0-700 400°-1300° SO42--SO2(g) 25 -1 9.2667 -7.4213 12.4 1.012487 Sakai (1968) SO42--H2S (aq) 25 3 14.009 -11.197 69.5 1.072008 Sakai (1968) SO42--H2S (aq) 25 5.07 0 6.33 63.4 1.065411 Robinson (1973) SO42--HS -(aq) 25 3 15.67 -13.592 72.7 1.075418 Sakai (1968) 25°- ~250°