S2. Detailed methods for stable isotope mixing model used to

S2. Detailed methods for stable isotope mixing model used to calculate dietary prey proportions.
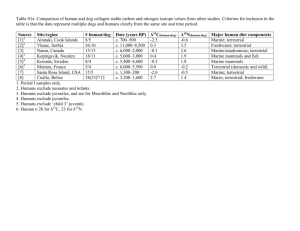
The mixing model, Stable Isotope Analysis in R (SIAR), was used to estimate dietary proportions (Parnell et al. 2010). Like earlier models (e.g., Phillips and Gregg 2003), SIAR can calculate absolute estimates of dietary proportions for n + 1 sources using n isotopes. For cases where sources are > n + 1, SIAR generates a range of possible combinations of source proportions that could produce observed consumer isotope values and reports mean proportion estimates as well as 95 % confidence intervals.
While diet-tissue discrimination is often assumed to be ~ 0.4 ‰ (Peterson and Fry 1987) for δ 13
C and 3.4 ‰ for δ
15
N (DeNiro and Epstein 1981; Minagawa and Wada 1984), discrimination factors vary among individuals (Gaye-Siessegger et al. 2004; Barnes et al. 2008), species (Macko et al. 1982), tissue types (Pinnegar and Polunin 1999), and diet types (Adams and Sterner 2000). For mixing model calculations, diet-tissue discrimination was assumed to be
1.4 ‰ for δ
13 C and 1.3 ‰ for δ 15
N based on available liver data for other fish species (Sweeting et al. 2007a; Sweeting et al. 2007b). To account for uncertainty and inherent variability in trophic discrimination values, standard deviation values of 1.8 ‰ and 1.4 ‰ were applied for
δ 13 C and δ 15
N, respectively, to approximate inter and intra-species variability (Barnes et al.
2008). Prey values were entered into SIAR models as mean values ± SD. When only a single prey sample was analyzed for a given prey group, standard deviation estimates of 0.40 ‰ were applied for each isotope to account for inter-individual variability. For samples from the Mid
Atlantic Bight, all liver samples were included in a single analysis within SIAR. For the Bay of
Biscay, liver samples were analyzed separately for summer and fall.
References
Adams TS, Sterner RW (2000) The effect of dietary nitrogen content on trophic level
15
N enrichment. Limnology and Oceanography 45: 601-607
Barnes C, Jennings S, Polunin N, Lancaster J (2008) The importance of quantifying inherent variability when interpreting stable isotope field data. Oecologia 155: 227-235
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et Cosmochimica Acta 45: 341-351
Gaye-Siessegger J, Focken U, Muetzel S, Abel H, Becker K (2004) Feeding level and individual metabolic rate affect δ 13 C and δ 15
N values in carp: implications for food web studies.
Oecologia 138: 175-183
Macko SA, Lee WY, Parker PL (1982) Nitrogen and carbon isotope fractionation by two species of marine amphipods - laboratory and field studies. Journal of Experimental Marine
Biology and Ecology 63: 145-149
Minagawa M, Wada E (1984) Stepwise enrichment of δ 15
N along food chains: further evidence and the relation between δ 15 N and animal age. Geochimica et Cosmochimica Acta 48:
1135-1140
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5: e9672
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annual Review of Ecology and
Systematics 18: 293-320
Phillips D, Gregg J (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136: 261-269
Pinnegar JK, Polunin NVC (1999) Differential fractionation of δ 13 C and δ 15
N among fish tissues: implications for the study of trophic interactions. Functional Ecology 13: 225-231
Sweeting C, Barry J, Barnes C, Polunin N, Jennings S (2007a) Effects of body size and environment on diet-tissue δ
15
N fractionation in fishes. Journal of Experimental Marine
Biology and Ecology 340: 1-10
Sweeting C, Barry J, Polunin N, Jennings S (2007b) Effects of body size and environment on diet-tissue δ
13
C fractionation in fishes. Journal of Experimental Marine Biology and
Ecology 352: 165-176