Amines - WordPress.com
advertisement

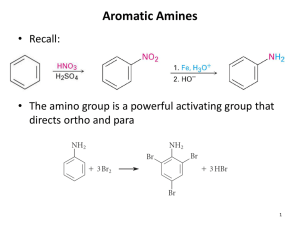
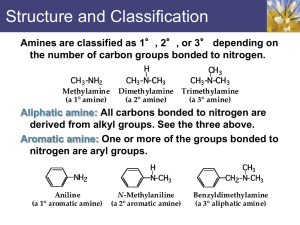
Amines 1.) Introduction Nitrogen is the fourth most common element in organic compounds (Carbon, Hydrogen, and Oxygen are the first 3) 2.) Structure and classifications a.) 1˚ Primary 2˚ Secondary 3˚ Tertiary b.) Aliphatic vs. Aromatic: If all carbons attached directly to the Nitrogen are alkyl groups (aliphatic hydrocarbons), the amine is an aliphatic amine. If one of the R groups attached to the nitrogen atom is aromatic-it is an aromatic amine. Ex: 3.) Nomenclature 4.) Physical properties of amines: a.) N-H……N hydrogen bond is weaker than the O-H…..O hydrogen bond Thus primary amines tend to have lower boiling points than primary alcohols of approximately the same MW. b.) All classes of amines are more water soluble than corresponding hydrocarbons of similar mol. Wt. c.) Higher molecular weight amines have lower solubility in water. 5.) Basicity : Amines are weak bases due to the availability of lone pair electrons on N. Basic Character in alkyl amines increases with increase in number of alkyl groups. However, the order of basicity in aqueous solution does not follow the expected trend and gets altered as revealed by their Kb values. The basic strength in aqueous solution depends not only upon electron releasing effect but also upon steric effect and hydration effect. Due to steric hindrance by three alkyl molecules in 30 amines the basicity decreases. Due to the resonance delocalisation of the lone pair into the aromatic π system the Aromatic Amines are less basic than aliphatic amines alkyl and non-aromatic heterocyclic amines are slightly stronger bases than ammonia. aryl amines are much weaker bases than ammonia, a result of the delocalisation of the lone pair into the π system of the ring. The basicity of aryl amines is: o o Increased by the presence of electrondonating substituents on the ring, by counter-acting the delocalisation of the lone pair into the πsystem of the ring. Decreased by the presence of electronwithdrawing substituents which enhance the delocalisation of the lone pair into the πsystem of the ring (especially those ortho or para to the amine functional group, see right). Preparations of Amines Alkylation of Ammonia Ammonia reacts as a nucleophile with alkyl halides to give primary amines in a nucleophilic substitution reaction. Yields are often poor as the product, a primary amine, RNH2, is itself a nucleophile and can react with more alkyl halide. The result are mixtures containing primary amines, secondary amines, tertiary amines and quaternary ammonium salts. This can be avoided if a large excess of ammonia is used. As aryl halides do not undergo simple nucleophilic substitution, they cannot be prepared using this method. Gabriel synthesis: The Gabriel synthesis uses the conjugate base of phthalimide (shown below) as a nitrogen nucleophile to displace a leaving group. The conjugate base is much more nucleophilic than phthalimide itself so the reaction stops cleanly after the addition of one alkyl group. The phthalimide can then be hydrolyzed in aqueous base to free the amine. The advantage of this method is that over alkylation is avoided (see previous page) Reaction of phthalimide with KOH removes the N-H proton giving an imide ion, a good nucleophile. Nucleophilic substitution by the imide ion on the alkyl halide generates an intermediate, N-alkyl phthalimide. Hydrolysis or hydrazinolysis liberates a primary alkyl amine. Aryl amines cannot be prepared via this method since aryl halides do not undergo simple nucleophilic substitution. Reduction of Azides Reduction of azides (N3): Sodium azide is a good source of the nucleophilic azide ion. This can be used to substitute for alkyl halides (alkyl azides are no longer nucleophilic) and the product can be reduced (commonly with lithium aluminum hydride followed with water). Reduction of Nitriles The nitrile, RC≡N, is reduced to the 1o amine by conversion of the C≡N to R-CH2-NH2 Reagents : either lithium aluminum hydride (LiAlH4) / ether solvent or catalytic hydrogenation (e.g. H2/Pd). Alkyl nitriles are prepared by nucleophilic substitution (SN2) by cyanide ion, CN-, of primary or secondary alkyl halides. Aryl nitriles can also be reduced to aryl amines. Reduction of nitriles: Sodium cyanide can be used to substitute a nitrile for a leaving group. The product can then be reduced (commonly with lithium aluminum hydride followed with water). Note that since the nitrile group contains a carbon, this adds one carbon in between the alkyl group with the leaving group and the amine. SN2 Reactions of amines with alkyl halides: This reaction is best used to prepare quaternary amines (ammonium salts) because an alkylamine is a better nucleophile than ammonia, and a dialkylamine is a better nucleophile than an alkylamine... The reaction cannot be cleanly stopped, so it is best used with an excess of alkyl halide to produce the quaternary amine. Reduction of amides: Amides can be reduced (again with lithium aluminum hydride) to form amines. Reductive amination: This process combines a reaction you've seen before - the nucleophilic attack of an amine at a carbonyl carbon - with reduction to remove the oxygen. An amine is mixed with a carbonyl compound in the presence of H2 and a metal catalyst. This results in the amine taking the place of the carbonyl group. This reaction can be used to prepare primary, secondary or tertiary amines. It is especially useful when different alkyl groups are needed on the nitrogen. (the reaction will be run several times, each time adding one of the desired alkyl groups) Arylamines are prepared by the reduction of nitrobenzenes. Several sets of reduction conditions are effective. (H2 with Pt, or Fe and HCl) Reactions of Amines Aliphatic amines are able to react: 1. as bases 2. as nucleophiles (mentioned above reaction with alkyl halides and discussed reacting with acid halides in chapter 21) 3. as precursors to leaving groups: The Hofmann Elimination reaction Note that neutral amines would have to become negative if they were to act as leaving groups. This is an even more unstable leaving group than hydroxide. The first step in turning an amine into a good leaving group is to let it react with an excess of methyl iodide to give the quaternary ammonium salt. This will then be neutral if it is pushed off as a leaving group. A base (Ag2O is a source of hydroxide ion and is traditionally used in this reaction) can then cause an elimination. The use of a large ammonium as the leaving group results in the LESS substituted alkene as the major product. This is unlike eliminations that use smaller leaving groups (such as a halide). Arylamines are able to react: 1. as substrates in electrophilic aromatic substitution. Two important considerations: 1. The -NH2 group is highly activating and multiply-substituted products are common 2. The -NH2 group forms a complex with some of the catalysts used (such as AlCl3 or FeCl3 and will prevent reactions using those catalysts These problems can be avoided by converting the arylamine into an arylamide (using reactions described in chapter 21), doing the desired electrophilic aromatic substitution, and then converting the amide back to an amine (by hydrolysis as described in chapter 21). 2. by conversion to diazonium salts (note that other amines can be converted to diazonium salts but the diazonium will usually decompose by loss of N2). The diazonium is the central structure in the following diagram. nucleophiles that can be used in diazonium replacement: HCl and CuCl HBr and CuBr NaI KCN and CuCN H3O+ H3PO2 nucleophile Br I CN OH H exact replacement Cl Problems Discussed In Class Problem 25.34 was tackled as an in-class problem. The problems indicates that prontosil should be synthesized starting from benzene. Results of Class Discussion The first thing to note about the desired product is that it appears to be the product of a diazonium coupling reaction. The diazonium coupling reaction requires that the diazonium react with an ACTIVATED ring. This helps decide the final step in the synthesis. The diazonium must start on the same ring as the sulfonamide as that is a deactivating group and will not couple if the diazonium is on the other ring. Here is the final step in the synthesis: This leaves us with two separate products that we will need to synthesize from benzene. Synthesis of the diazonium: The two substituents are para to each other. The sulfonamide is a deactivating substituent (because the sulfur has a partial positive charge) and will be a meta director. This substituent therefore cannot be put on first. Start the synthesis of this piece from benzene, and add the sulfonate when there is an ortho, para directing substituent (the amine in this case) in place. The final important note is that the diazonium should be formed before converting the sulfonyl chloride to the sulfonamide (otherwise the nitrogen on the sulfur will also be converted to the diazonium, which we would like to avoid). Here is the synthesis of the diazonium fragment: Synthesis of the diamine: An aryl amine is generated by reduction of a nitrobenzene. The amine is an o,p directing substituent whereas the nitro is a meta-directing substituent. The reduction should therefore be performed after the second nitro group is in place. Here is one possible synthesis of the diamine: