amines and amides note 2014 - SCH4U-SCHS
advertisement

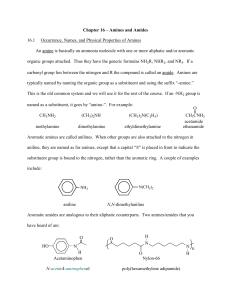
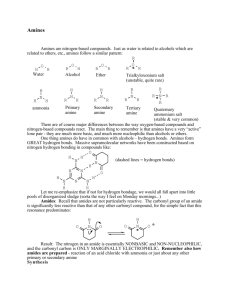
SCH 4U Amines An amine may be considered to be an ammonia molecule (NH3) in which one or more hydrogen atoms are replaced by alkyl groups. Amines may have one, two, or three alkyl groups attached to the nitrogen atom. These amines are c alled primary, secondary, and tertiary amines, respectively. Naming Amines 1. If the molecule has many substituents and the primary amine is of lower priority then number the carbon to which it is attached and use the prefix -amino then name it according to the parent chain. 2. To name secondary and tertiary amines, begin with an amine name based on the name of the longest alkyl group. Use the locator, N, to indicate the attachment of additional chains to the nitrogen atom, just as a number was used to indicate a specific carbon atom. 3. The IUPAC name for an aromatic amine consisting of an amine group attached to a benzene ring is aniline, rather than benzamine. Draw the following molecules2-amino-2-methylpropane 1,4-butyldiamine 1,5-pentyldiamine N-methyl-2-butylamine N,N-dimethyl-3-hexanamine N-phenylaniline 4-methyl-2-aminopentane 3-methoxyaniline N-ethyl-N-methylethanamine Aminocyclohexane aniline N-methyl aniline Properties of Amines Small amines (up to a 4 carbon chain) are soluble in water. Since nitrogen is more electronegative than either carbon or hydrogen, the N-C bonds and any N-H bonds are polar. Polarity is reduced in 2° and 3° amines. 1° and 2° amines have higher melting points and higher boiling points than corresponding hydrocarbon compounds due to hydrogen bonding. They have pyramidal geometry around the central N atom due to the lone pair of electrons. Amines are weak bases because the unshared electron pair of the nitrogen atom can form a coordinate covalent bond with a proton (H+). Ex. RNH2 (aq) + H2O (l) RNH3+ (aq) + OH-(aq) They often have offensive odours. Ex. See putrescine and cadaverine are amines produced in protein decomposition. Reactions Involving Amines 1. Synthesis of 1°, 2° and 3° amines using an haloalkanes (alkyl halides) The limitation to this method is the creation of a mixture of the amines as products. This mixture must be separated by distillation. Alkyl halide and ammonia Alkyl halide and primary amine Alkyl halide and secondary amine 2. Synthesis of an amide (Condensation reactions of carboxylic acids) 3. Combustion 4. Neutralization reactions Amines react with acids to produce ammonium salts Amides Amides are structurally similar to esters, except the two chains are joined by a nitrogen atom next to a carbonyl group rather than by an oxygen atom. This is a result of the reaction of an acid with an amine. The amide functional group consists of a carbonyl group bonded to a nitrogen atom. Naming Amides Naming amides is similar to naming esters. While esters end in –oate, amides end in –amide. The name of an amide has two parts: 1. One part of the name comes from the acid. Locate the part of the amide that contains the carbonyl group. Name this portion as an acid but replace the –oic ending with –amide. 2. Decide whether the amine portion of the amide is primary, secondary or tertiary. 3. If the amide contains a primary N, no other prefixes are needed. 4. If the N is secondary or tertiary the naming is similar to amines but shortened to end in –yl. Propanamide Pentanamide N-ethylpentanamide N-isopropyl-N-methylbutanamide N,N-dipropylethanamide benzamide 2-hydroxybenzamide Properties of Amides The smaller amides are soluble in water. This can be explained by the molecules’ structure: the –NH2 groups form hydrogen bonds with neighbouring molecules. As the length of the carbon chain increases, solubility decreases. Primary amides, with their nitrogen atoms each bonded to 2 hydrogen atoms, have higher melting points and boiling points than similar amides. This is likely due to more hydrogen bonding between primary amide molecules. Like amines, amides are weak bases. Reactions Involving Amides 1. Synthesis of Amides (Condensation Reaction) The reaction of ammonia with acid chlorides or acid anhydrides 2. Hydrolysis In acidic or basic conditions, amides can be hydrolyzed to produce a carboxylic acid and an amine. This reaction is essentially the reverse of the formation reactions of amides above. This reaction proceeds slowly which is important to the stability of proteins. 3. Protein Synthesis (Condensation Reaction) The building blocks of proteins are called amino acids. When amino acids react they form long chains called proteins.