Stereochemistry - chemistry
advertisement

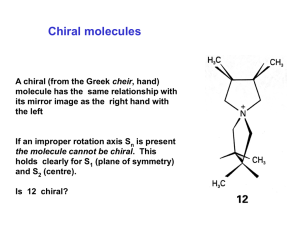
CHE 240 Unit IV Stereochemistry, Substitution and Elimination Reactions CHAPTER FIVE Terrence P. Sherlock Burlington County College 2004 Chiral Carbons • Tetrahedral carbons with 4 different attached groups are chiral. • Its mirror image will be a different compound (enantiomer). => Chapter 5 2 Mirror Planes of Symmetry • If two groups are the same, carbon is achiral. (animation) • A molecule with an internal mirror plane cannot be chiral.* Caution! If there is no plane of symmetry, molecule may be chiral or achiral. See if mirror image can be superimposed. => Chapter 5 3 (R), (S) Nomenclature • Different molecules (enantiomers) must have different names. • Usually only one enantiomer will be biologically active. O OH C • Configuration around the chiral carbon is specified H3C C H NH2 with (R) and (S). n atu ral al an i n e Chapter 5 => 4 Cahn-Ingold-Prelog Rules • Assign a priority number to each group attached to the chiral carbon. • Atom with highest atomic number assigned the highest priority #1. • In case of ties, look at the next atoms along the chain. • Double and triple bonds are treated like bonds to duplicate atoms. => Chapter 5 5 Assign (R) or (S) • Working in 3D, rotate molecule so that lowest priority group is in back. • Draw an arrow from highest to lowest priority group. • Clockwise = (R), Counterclockwise = (S) Chapter 5 => 6 Properties of Enantiomers • • • • Same boiling point, melting point, density Same refractive index Different direction of rotation in polarimeter Different interaction with other chiral molecules – Enzymes – Taste buds, scent => Chapter 5 7 Optical Activity • Rotation of plane-polarized light • Enantiomers rotate light in opposite directions, but same number of degrees. => Chapter 5 8 Polarimetry • • • • • Use monochromatic light, usually sodium D Movable polarizing filter to measure angle Clockwise = dextrorotatory = d or (+) Counterclockwise = levorotatory = l or (-) Not related to (R) and (S) Chapter 5 => 9 Biological Discrimination => Chapter 5 10 Racemic Mixtures • • • • Equal quantities of d- and l- enantiomers. Notation: (d,l) or () No optical activity. The mixture may have different b.p. and m.p. from the enantiomers! => Chapter 5 11 Racemic Products If optically inactive reagents combine to form a chiral molecule, a racemic mixture of enantiomers is formed. => Chapter 5 12 Nonmobile Conformers If the conformer is sterically hindered, it may exist as enantiomers. => Chapter 5 13 Fischer Projections • Flat drawing that represents a 3D molecule • A chiral carbon is at the intersection of horizontal and vertical lines. • Horizontal lines are forward, out-of-plane. • Vertical lines are behind the plane. Chapter 5 14 Fischer Rules • Carbon chain is on the vertical line. • Highest oxidized carbon at top. • Rotation of 180 in plane doesn’t change molecule. • Do not rotate 90! • Do not turn over out of plane! => Chapter 5 15 Fischer Mirror Images • Easy to draw, easy to find enantiomers, easy to find internal mirror planes. • Examples: CH3 CH3 CH3 H Cl Cl H H Cl Cl H H Cl H Cl CH3 CH3 CH3 Chapter 5 => 16 Fischer (R) and (S) • Lowest priority (usually H) comes forward, so assignment rules are backwards! • Clockwise 1-2-3 is (S) and counterclockwise 1-2-3 is (R). • Example: (S) CH3 (S) H Cl Cl H CH3 Chapter 5 => 17 Diastereomers • Stereoisomers that are not mirror images. • Geometric isomers (cis-trans) • Molecules with 2 or more chiral carbons. => Chapter 5 18 Alkenes Cis-trans isomers are not mirror images, so these are diastereomers. H H H C C C C H3C CH3 CH3 cis-2-bu te n e H3C H trans-2-bu te n e Chapter 5 => 19 Ring Compounds • Cis-trans isomers possible. • May also have enantiomers. • Example: trans-1,3-dimethylcylohexane CH3 CH3 H H H => H CH3 CH3 Chapter 5 20 Two or More Chiral Carbons • Enantiomer? Diastereomer? Meso? Assign (R) or (S) to each chiral carbon. • Enantiomers have opposite configurations at each corresponding chiral carbon. • Diastereomers have some matching, some opposite configurations. • Meso compounds have internal mirror plane. • Maximum number is 2n, where n = the number of chiral carbons. => Chapter 5 21 Examples COOH COOH H HO HO OH H H H OH COOH COOH (2S,3S)-tartaric aci d (2R,3R)-tartaric aci d COOH H OH H OH COOH (2R,3S)-tartaric acid Chapter 5 => 22 Fischer-Rosanoff Convention • Before 1951, only relative configurations could be known. • Sugars and amino acids with same relative configuration as (+)-glyceraldehyde were assigned D and same as (-)-glyceraldehyde were assigned L. • With X-ray crystallography, now know absolute configurations: D is (R) and L is (S). • No relationship to dextro- or levorotatory. => Chapter 5 23 D and L Assignments CHO H * CHO OH H CH2OH D-(+)-glyce ral de h yde HO H COOH H2N H OH H OH => H * OH CH2CH2COOH CH2OH L-(+)-gl u tam i c aci d D-(+)-gl u cose * Chapter 5 24 Properties of Diastereomers • Diastereomers have different physical properties: m.p., b.p. • They can be separated easily. • Enantiomers differ only in reaction with other chiral molecules and the direction in which polarized light is rotated. • Enantiomers are difficult to separate. => Chapter 5 25 Resolution of Enantiomers React a racemic mixture with a chiral compound to form diastereomers, which can be separated. Chapter 5 => 26 Chromatographic Resolution of Enantiomers => Chapter 5 27 POWER POINT IMAGES FROM “ORGANIC CHEMISTRY, 5TH EDITION” L.G. WADE ALL MATERIALS USED WITH PERMISSION OF AUTHOR PRESENTATION ADAPTED FOR BURLINGTON COUNTY COLLEGE ORGANIC CHEMISTRY COURSE BY: ANNALICIA POEHLER STEFANIE LAYMAN CALY MARTIN Chapter 5 28