Hot Topics in Translational Research: Transforming Cutting
advertisement

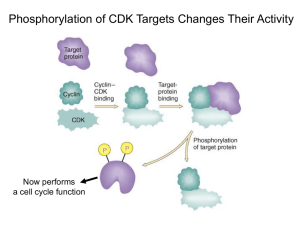
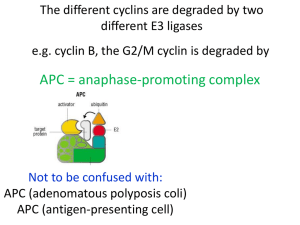
Antireplication Agents: CDK Inhibitors and Telomerase Inhibitors Richard S. Finn, MD Assistant Professor of Medicine Geffen School of Medicine at UCLA Faculty Disclosure Richard Finn, MD, has disclosed that he has received fees for non-CME services from Genentech. Cyclin D Kinases and Cancer CDKs are a subgroup of seine/ threonine kinases In general very small proteins (34-40 kDa) Bind to activating proteins: cyclins Without cyclins, CDKs have little kinase activity Play a key role in regulating cell cycle progression through all phases of the cell cycle Various cyclin/ CDK complexes act at different parts of the cell cycle Temporal and quantitative regulation Negative regulation by cyclin dependent kinase inhibitors (CKI) INK 4 family (p15, p16, 18, p19 Cip/ Kip family (p21, p27, p57) Alterations in CDKs are uncommon, compared with cyclin dysregulation Altered regulation/ expression in many malignaices Cyclin D1 amplification has been described in various malignancies, including breast cancer, with variable prognostic significance t(11;14) mantle cell lymphoma Rb loss, a well known oncogenic event 1. Finn RS, et al. Breast Cancer Res. 2009;11:R77. 2. Lundberg AS, et al Eur J Cancer. 1999;35:1886-1894.3. Buckley MF, et al. Oncogene. 1993;8:2127-2133. 4. Dickson C, et al. Cancer Lett. 1995;90:43-50. Cyclin D Kinases and Cancer Vermullen Cell Proliferation Rb as Master-Regulator of the R Point p16(INK4a) Modified from Figure 8.19 . The Biology of Cancer. © Garland Science 2007. CDK/ Cyclin Inhibitors Targeting CDK >50 inhibitors described Generally in several classes – Purine analogoues (ie roscovitine) Selicicilib (CYC202) CDK2/E CDk 2/A CDK7 CDK 9 – Pyrimidine analgoues Dinaciclib (SCH727965) CDK 2, CDk5, CDK 1, CDK 9 – Flavonoids (flavopiridol) – Indolinones – Staurosporine Targeting Cyclin cyclin expression modulators (ON013105) CDK/ Cyclin Inhibitors Selicicilib (CYC202) – Phase II Nasopharyngeal APPRAISE Study Randomized Phase II, NSCLCA 3rd line or greater vs BSC Dinaciclib (SCH727965) Phase II – Melanoma – AML ON013105 Phase I- mantle cell lymphoma PD 0332991: Background O The compound N Potent, selective, reversible inhibitor of HN CDK4,6 Small molecule Oral agent N O N O N OH S O + The opportunity N O N H2 Potential first in class Potential impact on hematopoietic and solid tumors Potential use in pediatric indications Single-agent and combination approaches under investigation Finn RS, et al. Breast Cancer Res. 2009;11:R77. PD 332991 Rb as Master-Regulator of the R Point Target of PD 0332991 p16(INK4a) Inactivates Rb and allows progression Modified from Figure 8.19 . The Biology of Cancer. © Garland Science 2007. Sorlie et al PNAS 2001 Human Breast Cancer Cell Line Panel Can Recapitulate the Molecular Heterogeneity of Clinical Disease 51 Human Breast Cell Lines 25 Luminal 10 9 6 ER positive ER positive ER negative Normal HER-2 HER-2 amplified HER-2 amplified 26 Non-luminal 4 Non-malignant 13 Basal/Progenitor 9 Mesenchymal 1 HER-2 Amplified 1 HER-2 Amplified M BZR 175 75 CA 30 M A M -1 B1 HC 34 UA C2 CC 02 -8 9 EF 3 M SU 19 M EF 190 M 19 M 2A BHC 36 C1 1 HC 50 C1 0 41 HC 9 C M 38 BM 415 CF U A -1 0 CC A HC -81 C2 2 2 ZR 18 M DA 75M 1 B4 5 18 3 4A 1 T4 7D M CF 7 B M T DA -2 M 0 B4 3 BT 5 47 SK 4 BR KP 3 HC L1 M C1 DA 14 3 M B HC 23 C1 1 SU 395 M -2 HS 25 57 8 18 T UA 4B CC 5 7 CA 32 L5 BT 1 CO 54 LO 9 DU 824 4 HC 47 C1 5 HC 18 C1 7 HC 56 C1 9 HC 80 C 6 HC 193 C1 7 95 HC 4 C M 70 B43 M 6 M B1 DA 5 M 7 B4 68 PD-0332991: CDK 4/6 Inhibitor: Breast Panel 1000 900 800 700 600 500 400 300 200 100 0 Subtype Luminal HER2 Amplified Immortalized Non-luminal/post EMT Non-luminal PD 0332991: Cell Cycle Analysis MCF7 % E FM192A H C C 1419 100 100 100 90 90 90 80 80 80 70 70 70 60 60 60 50 50 50 40 40 40 30 30 30 20 20 20 10 10 10 0 0 G 0/G 1 S G2 0 G 0/G 1 S G2 G 0/G 1 S G2 Sensitive lines H C C 1937 % H C C 1187 MD A MB 468 100 100 100 90 90 90 80 80 80 70 70 70 60 60 60 50 50 50 40 40 40 30 30 30 20 20 20 10 10 10 0 0 G 0/G 1 S G2 0 G 0/G 1 S Resistant lines Finn RS, et al. Breast Cancer Res. 2009;11:R77. G2 G 0/G 1 S G2 PD 0332991: Effects on Phosphorylation on Retinoblasoma Gene Product A. Total pRb Time 0 30’ 60’ 12 hr 24 hr B. Phospho-Rb (serine 780) 48 hr 0 30’ 60’ 12 hr 24 hr 48 hr MCF7 MB453 Sensitive T47D EFM19 HCC1187 HCC1954 CAL 51 Finn RS, et al. Breast Cancer Res. 2009;11:R77. Resistant Hypothesis: Patient Selection in Breast Cancer Population Elevated Cyclin D1 RB Decreased p16 Gauthier ML, et al. Cancer Cell. 2007;12:479-491. 100 2 60 CI MCF7 % Inhibition 80 1 40 20 0 0 Tam PD 10000 100 5000 2500 50 1250 25 625 12.5 6.25 1000 5000 2500 1250 625 312.5 100 50 25 12.5 6.25 3.125 312 3.125 Concentration (nM) Concentration (nM) 2 100 60 CI EFM19 % Inhibition 80 1 40 20 0 0 Tam PD 5000 2500 50 25 1250 625 312 12.5 Concentration (nM) 6.25 3.125 Tam 5000 2500 PD 50 25 1250 625 312 12.5 6.25 3.125 Concentration (nM) 100 2 Combo PD-2991 Tamoxifen 60 CI % Inhibition 80 T47D 1 40 20 0 Tam PD 5000 2500 1250 625 312 50 25 12.5 Concentration (nM) 6.25 3.125 0 Tam 5000 2500 1250 625 312 PD 50 25 12.5 6.25 3.125 Concentration (nM) TRIO 18/A5481003: Phase I/II Study of Letrozole in Combinations With PD-0332991 in Postmenopausal ER+ Advanced Breast Cancer Phase I complete Randomized phase II accruing ClincailTrials.gov. NCT00721409. TRIO 18: Phase I Patient Summary Slamon DJ, et al. ASCO 2010. Abstract 3060. TRIO 18: Most Common AEs (N = 12) PT, n Grade 1 Grade 2 Grade 3 Grade 4 Total Neutropenia 0 1 7 2 10 Fatigue 6 2 0 0 8 Leukopenia 0 3 2 1 6 Nausea 5 0 0 0 5 Diarrhea 4 0 0 0 4 Anemia 2 1 0 0 3 Cough 2 1 0 0 3 Decreased appetite 3 0 0 0 3 Dyspnea 3 0 0 0 3 Hot flush 2 1 0 0 3 Nasal congestion 2 1 0 0 3 Arthralgia 1 1 0 0 2 Back pain 2 0 0 0 2 Creatinine increased 0 1 1 0 2 Slamon DJ, et al. ASCO 2010. Abstract 3060. TRIO 18: Most Common TreatmentRelated AEs (N = 12) PT, n Grade 1 Grade 2 Grade 3 Grade 4 Total Neutropenia 0 1 7 2 10 Fatigue 4 2 0 0 6 Leukopenia 0 3 2 1 6 Nausea 5 0 0 0 5 Anemia 2 1 0 0 3 Decreased appetite 3 0 0 0 3 Diarrhea 3 0 0 0 3 Hot flush 2 1 0 0 3 Dyspnea 2 0 0 0 2 Headache 1 1 0 0 2 Thrombocytopenia 1 1 0 0 2 Slamon DJ, et al. ASCO 2010. Abstract 3060. TRIO 18: Phase I Summary Phase I (N = 12) • MTD: PD 0332991 125 mg QD (schedule 3/1) in combination with letrozole 2.5 mg QD • 3 DLTs: – 2 patients with grade 4 neutropenia – 1 patient with 5 doses held due to elevated creatinine deemed treatment related • No treatment-related SAEs • No discontinuations due to AEs – Common treatment-related AEs: neutropenia, leukopenia, fatigue • No febrile neutropenia • No drug-drug interaction • Efficacy: 3 PRs and 9 SDs (PR: 33%; CBR: 67%) • Median duration of treatment: 12 mos (range: 2-21+) • Currently 6 patients active Slamon DJ, et al. ASCO 2010. Abstract 3060. Hypothesis: Biomarkers Predictive of PD 0332991 Sensitivity Desired biomarker profile: ER+, HER2– Wild-type Rb1 – Plus • Amplified cyclin D1/CCND1 OR • Inactivated CDKN2A/p16INK4a Finn RS, et al. Breast Cancer Res. 2009;11:R77. Phase II Study Design (Part I, Completed) ER+, HER2- breast cancer Stratification Factors: Disease site − Visceral vs bone only vs other Disease-free interval − > 12 vs ≤ 12 mos R A N D O M I Z A T I O N N = 60 Primary endpoint: PFS ClincailTrials.gov. NCT00721409. Arm A PD 0332991 125 mg/day (Schedule 3/1) + Letrozole 2.5 mg/day 1:1 Arm B Letrozole 2.5 mg/day Phase II Study Design (Part II, Ongoing) ER+, HER2- breast cancer Biomarker Selection CCND1 amp And/or loss of p16 R A N D O M I Z A T I O N N = 150 Primary endpoint: PFS ClincailTrials.gov. NCT00721409. Arm A PD 0332991 125 mg/day (Schedule 3/1) + Letrozole 2.5 mg/day 1:1 Arm B Letrozole 2.5 mg/day Similar Observations in Other Histologies Ovarian cancer[1] Glioblastoma[2,3] Multiple myeloma[4] 1. Konecny GE, et al. Clin Cancer Res. 2011;17:1591-1602. 2. Michaud K. Cancer Res. 2010;70:32283238. 3. Wiedemeyer WR, et al. Proc Natl Acad Sci U S A. 2010;107:11501-11506. 4. Menu E, et al. Cancer Res. 2008;68:5519-5523. Telomerase and Cancer Telomerase: reverse transcriptase that adds DNA repeats (TTAGGG) to the 3’ end of DNA strands (telomere region) – Consists of 2 molecules each of telomerase reverse transcriptase (TERT), telomerase RNA (TERC), and dyskerin (DKC1) Protects DNA from genomic damage/loss during replication In cancer, cells that lose telomeres become unstable, accumulate genetic damage, and eventually undergo apoptosis Activation of telomerase can prevent the apoptosis event and cause cells to become immortalized – Telomerase is activated in 90% of cancer (but not somatic cells) Hypotheses: block telomerase, induce telomere shortening, genetic instability and cell death Shay JW, et al. Human Mol Gen. 2001;10:677-685. Telomerase MOA Harley Nat rev Cancer 2008 Telomerase and Cancer Various potential methods of silencing telomerase – Oligonucleotides • Target the template region (activation site) of telomerase • Imetelstat (GRN163L): 13 mer oligonucleotide, not antisense but a direct telomerase inhibitor[1] – Vaccines • Dendritic cell–based (GRNVAC1) • Use hTERT pulsed autologous dendritic cells • Phase I: generally well tolerated, achieved levels felt to be sufficient for hTERT inactivation[2] • Phase II study in AML as consolidation • Nondendritic based • Phase I in solid tumors (V934/V935)[4] • GV1001 peptide with temozolomide for melanoma[5] 1. Herbert BS, et al. Oncogene. 2005;24:5262-5268. 2. Su Z, et al. J Immunol. 2005;174:3798-3807. 3. ClinicalTrials.gov. NCT00510133. 4. ClinicalTrials.gov. NCT00753415. 5. ClinicalTrials.gov. NCT01247623. Conclusions Newer targets in molecular oncology include the CDK pathway and telomerase Ongoing clinical studies (TRIO18) with PD 0332991, an oral, small molecule kinase inhibitor, in breast cancer aim at validating laboratory science that identified ER+ breast cancer as being susceptible to CDK 4/6 inhibition To date, predictable and manageable toxicity has been seen with this class of agent Studies with other CDK targeted agents and Cyclin targeted agents are ongoing MCL should be “proof of concept” Telomerase inhibitors are moving to the clinic and hold promise but predictors of response will be necessary for successful clinical development Including vaccine strategies and short-oligos