Cell Cycle II
advertisement

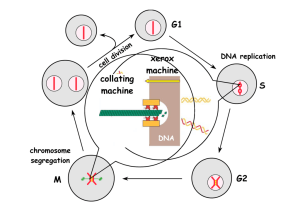
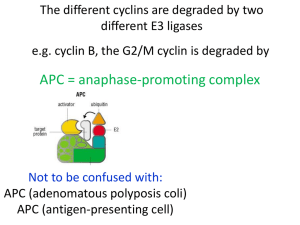
Phosphorylation of CDK Targets Changes Their Activity Now performs a cell cycle function Yeasts have one CDK and several cyclins Humans have 4 CDKs and 4 cyclins CDK/Cyclin complex regulation controls the cell cycle X - Cyclin-Cdk complexes function in different phases - G1/S-Cdk complexes commit the cell to a new cell cycle - S-Cdk complexes promote S phase (and block it in G2!) - M-Cdk complexes trigger entry into mitosis - M-Cdk complexes are inactivated before anaphase Example: cycB-Cdk1 appears in mitosis, phosphorylates lamin and leads to nuclear envelope breakdown during early mitosis cycB-Cdk1 will be destroyed during mitosis to allow formation of a new nuclear envelop breakdown during telophase How are CDK’s Regulated? 1. By cyclin synthesis and destruction 2. By phosphorylation 3. By binding to CDK inhibitory proteins (CKIs) Generation of a “Cycling” Frog Egg Extract 1. Inject females with hormones so that they lay eggs 2. Pack eggs into a centrifuge tube and spin 4. Add sperm chromatin and away you go! 3. Remove Cytoplasmic Extract Cyclin Synthesis and Destruction Is Essential for Cell Cycle Progression sea urchin! sea urchin! Cyclin Destruction is Controlled by Ubiquitylation But what is ubiquitylation??!! Ubiquitylation: a post-translational protein modification E3 ubiquitin ligase is a protein complex that confers specificity: i.e. which protein to target Proteasome: the cellular garbage can E1, E2, and E3 enzyme cascade An E3 ubiquitin ligase called the Anaphase Promoting Complex (APC) destroys mitotic cyclins (and other things) Not to be confused with: APC (adenomatous polyposis coli) APC (antigen-presenting cell) How does the APC function? M APC M Cyclin and securin must be destroyed in order for anaphase to take place APC Activity changes during the cell cycle Negative feedback generates a repeating oscillator The Cell Cycle According to Oscillating Cyclin/CDK and APC Activity A cyclin promotes synthesis of the next cyclin that in turn, promotes destruction of the previous one Once and Only Once S phase is Controlled by CDKs The Cell Cycle According to Oscillating Cyclin/CDK and APC Activity But is cyclin abundance the only way to control CDK activity? NO! How else are CDKs Regulated? Genetic studies in fission yeast. CDKs are Regulated by Phosphorylation is a kinase is a phosphatase CAK (CDK Activating Kinase) Conformational Changes Associated with CDK Phosphorylation Free CDK The T-loop blocks substrate access CDK + Cyclin Binding of cyclin moves the T-loop T161 phosphorylation Phosporylation moves the T-loop more How else are CDKs Regulated? Biochemical studies in mammalian cells. Figure 8.8 The Biology of Cancer (© Garland Science 2007) CDKs are regulated by Cyclin Dependent Kinase Inhibitors (CKIs) p21 CDK CDK Cyclin Cyclin p16 CDK4 Cyclin CDK4 p16 p21 The Discovery of p21 and p16: what binds to CDKs? Adding Transformed Transformed 35S[Met] Cell Line Normal Cultured cells Normal a-CDK4 Competing peptide - + - + CDK4 Metabolic labeling Cyclin p21 Lysis cells midly Add anti-CDK4 antibody Add protein A-agarose beads Immunoprecipitate SDS-PAGE Cyclin D CDK4 CDK4 p16 Autoradiography p21 p16 Xiong et al. (1993) Genes & Dev. 7:1572 The p21 Family of CDK inhibitors (p21CIP1/WAF1, p27KIP1, p57KIP2) active inactive CDK CDK Cyclin + p21 Cyclin p21 Jeffrey et al. (1995) Nature 376:313 Figure 8.13b The Biology of Cancer (© Garland Science 2007) Russo et al. (1996) Nature 382:325 The INK4 Family of CDK inhibitors (p16INK4a, p15INK4b, p18INK4c, p19INK4d) active inactive CDK4/6 CDK4/6 Cyclin D + Russo et al. (1998) Nature 395:237 Brotherton et al. (1998) Nature 395:244 INK4 INK4 + Cyclin D CKIs Regulate the G1-S Transition (p16) (p21, p27) p16 is Frequently Mutated in Human Tumors Table 1. D eleti ons i n tumor cells and p rimary tumors. 9p21 Tumor t ype L ines (n) Del etion s (n) D eleti ons (%) Astrocytoma Bladder Breast Colon G lioma L eukemia L ung Melan oma N euro blast oma O steosarcoma O vary Renal 17 15 10 20 35 4 59 99 10 5 7 9 14 5 6 0 25 1 15 57 0 3 2 5 82 33 60 0 71 25 25 58 0 60 29 56 Total 29 0 13 3 46 See Kamb et al. (1994) Science 264: 436; Nobori et al. (1994) Nature 368:753 for detail Therapeutic Targeting of the Hallmarks of Cancer Hanahan and Weinberg, Cell 144:646 (2011) Chemical structures of small molecular cdk inhibitors Senderowicz, A. M. et al. J Natl Cancer Inst 2000;92:376-387 Table 1. Pharmacologic effects of flavopiridol* Effect Growth inhibition, NCI DTP screen cdk inhibition Apoptosis IC50, nM 66 40-200 100-1000 Cell cycle arrest 100-300 Cyclin D1 depletion 100-300 Differentiation 100-300 VEGF depletion Sensitization to standard chemotherapies Epidermal growth factor receptor tyrosine kinase inhibition Protein kinase A inhibition 50-100 100-300 21 000 122 000 •IC50 = concentration that inhibits growth or activity by 50%; •NCI = National Cancer Institute; DTP = Developmental Therapeutics Program; VEGF = vascular endothelial growth factor. Senderowicz, A. M. et al. J Natl Cancer Inst 2000;92:376-387 Table 2. Phase I trials with cdk modulators Flavopiridol (96) UCN-01 (131) Schedule 72-h continuous infusion every 2 wk 72-h continuous infusion every 4 wk (cycle 1) followed by 36h continuous infusion every 4 wk (cycles 2 or higher) Dose-limiting toxicity (maximal tolerated dose) Diarrhea (50 mg/m2 per day for 3 days) Nausea/vomiting, hyperglycemia, and hypoxemia (42.5 mg/m2 per day for 3 days) Hypotension (78 mg/m2 per day for 3 days) Other toxic effects Anorexia, proinflammatory syndrome Headache, myalgias Suggestion of activity Non-Hodgkin's lymphoma and renal, colon, gastric, or prostate cancer Melanoma, non-Hodgkin's lymphoma, or leiomyosarcoma Median plasma concentration at maximal-tolerated dose 271 nM (50 mg/m2 per day for 3 days) 344 nM (78 mg/m2 per day for 3 days) Total = 36.4 µM (42.5 mg/m2 per day for 3 days) Free* = 111 nM (42.5 mg/m2 per day for 3 days) Plasma half-life, h 11.6 588 * Free = concentration of UCN-01 in saliva. Senderowicz, A. M. et al. J Natl Cancer Inst 2000;92:376-387 http://www.clinicaltrials.gov/ 51 studies for CDK inhibitors