Introduction to the Cell Cycle
advertisement

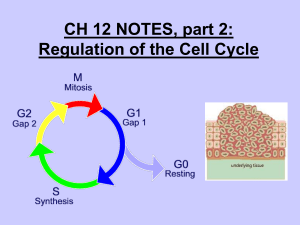
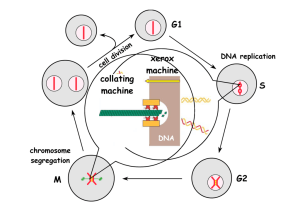
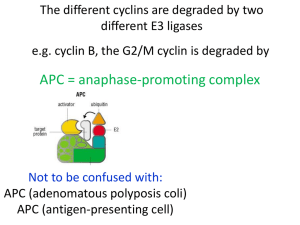
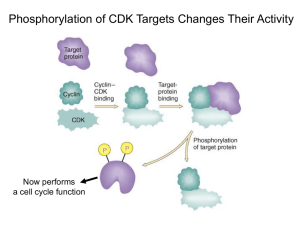
Introduction to the Cell Cycle Promega Education Resources Unit 7 Overview • In order for adult multicellular organisms to develop from a single fertilized egg, cell growth and division has to occur at the appropriate times and in the appropriate places. • When cell cycles proceed inappropriately (e.g., cells divide uncontrollably), pathological conditions like cancer can result. • Cells must accomplish two basic things during the cell cycle: Copying cellular components Dividing the cell so that components are distributed evenly to the daughter cells • The alternating “growth” and “division” activities of the cell is called the “cell cycle”. • The division activity corresponds to “M phase”. • The “growth” activity corresponds to “Interphase”. Interphase • Interphase can be subdivided into G1, S and G2 phases. In yeast “Start” is at the end of G1; at this point the cell is committed to DNA synthesis. In mammals, this is called the “restriction point”. This point late in G1 is a “checkpoint”; a cell will exit the cell cycle if certain requirements to proceed to synthesis are not met. A second restriction point occurs in G2 before entry into mitosis. Interphase G1 S M G2 M Interphase G1 S G2 M M Interphase G1 S G2 Interphase: G1 • Events during G1 Cell growth Preparation of chromosomes for replication Duplication of cellular components G1 checkpoint (or restriction point); cell commits to division or exits from cell cycle Interphase: S phase • DNA replication • Duplication of the centrosome The centrosome is located near the nucleus of the cell and contains the microtubule organizing center MTOC in animal cells. It contains two centrioles that migrate to the poles before cell division and serve to organize the spindle. Interphase: G2 • Cell growth • Checkpoint (restriction point) for entry into M phase M phase • Cell division (mitosis or meiosis for germ cells) • Can be subdivided into four subphases: Prophase Metaphase Anaphase Telophase • Factors that influence M phase entry Cellular Mass Growth Rate Time (During early embryogenesis, divisions may proceed rapidly, essentially alternating M and S phases, with little growth between them.) Completion of DNA Replication Studying the Cell Cycle • Much of what we know about the cell cycle comes from the study of model experimental systems: Genetics • Yeast: Schizosaccharomyces pombe (fission yeast) and Saccharomyces cerevisae (budding yeast) • Short article from HHMI (http://www.hhmi.org/genesweshare/a300.html) Biochemistry • Frog eggs • Mammalian cell culture Control of the Cell Cycle • Intrinsic Control: Cyclins Proteins whose levels rise and fall during the various phases of the cell cycle (primary regulators of the cyclin-dependent kinases) Interact with the cyclin-dependent kinases (cdk) Cdk levels are constant Cdks must bind to cyclins to be activated The complexes of cyclin+cdk act in concert. The cdks phosphorylate proteins that initiate or regulate cell cycle activities. Cyclins also may be involved in cdk target recognition. Cdk activity is terminated by cyclin degradation (generally). Cdk activity can be “fine tuned” by other mechanisms (i.e., inhibitory phosphorylation by Wee1 kinase, activation by cdc25 kinase. Cdk inhibitor proteins also can regulate the cyclin-cdk complexes. Cyclins • Four classes Defined by phase of the cell cycle in which they bind their cdk •G1/S phase cyclins-bind cdks at the end of G1, commit cell to DNA replication (cyclin E) •S phase cyclins- bind cdks during S phase, required to initiate replication (cyclin A) •M phase cyclins- bind cdks immediately before M phase, initiate early mitotic (or meiotic) events (cyclin B) •G1 cyclins- involved in progression through the checkpoint in late G1 (cyclin D) M phase Promoting Factor (MPF) • Cytoplasmic protein factor responsible for initiation of meiotic and mitotic phases of cell cycles in eukaryotes • First detected in unfertilized amphibian eggs • Progesterone triggers oocyte maturation; MPF is important for progression from meiosis I) • MPF activity rises at beginning of meiosis II and before mitotic divisions after fertilization • MPF activation, oocyte activation and mitosis will not occur without protein synthesis* What is MPF? • MPF is a complex of proteins • In 1988, one of the proteins associated with MPF was shown to be homologous to the cdc2 gene of S. pombe • cdc2 gene encodes a protein kinase • Could one of the other components be cyclins? Xenopus oocyte maturation • Progesterone triggers oocyte maturation • Oocytes progress through two cycles of mieoisis, but the second meiotic division is arrested at metaphase II until fertilization • Oocytes are released from metaphase II arrest when exposed to sperm chromatin • Scientists often prepare extracts from oocytes that have been released from metaphase II arrest for their studies. • Cytoplasm extracted from oocytes will “cycle” when exposed to sperm chromatin Key Experiment • Murray and Kirschner (1989) • Determine that cyclin is involved (isolated “cycling” proteins from extract) • Show cyclin synthesis is required to drive cell cycle • Prepare extracts from unfertilized Xenopus eggs Contain all materials required for multiple cell cycles Can synthesize proteins from maternal mRNA in the cytoplasm Can be mixed with chromatin from interphase sperm generates a haploid nucleus with sperm DNA, which will replicate, and the extract will go through “mitosis” (cycling extracts). Experimental Details • Treat cycling extracts with limited amount of RNase A Degrades all of the maternal mRNAs Does not degrade the tRNAs or rRNAs necessary for protein synthesis (these RNAs are protected by proteins within the protein/RNA complexes) • Add RNasin Ribonuclease Inhibitor to inactivate RNase A • Add back cyclin B mRNA Extracts resume cycling activity • Add cyclin B mRNA that encodes a protein with a mutated protein-degradation signal to parallel extracts Extracts start to cycle, but get “stuck” in early mitotic events Conclusion: Cyclin B synthesis and degradation are necessary for cycling. Cell Cycle Checkpoints • The decision to proceed from one part of the cell cycle to another depends on a variety of factors Growth DNA replication DNA integrity Cellular integrity The mechanisms that the cell has to monitor these factors act at “checkpoints” Generally, the feedback from checkpoints is through negative regulation—sending a signal to stop the progression of the cell cycle rather than dialing back a positive signal. Schematic of Cell Cycle Checkpoints Cell Cycle Checkpoints • G1 (Restriction) Checkpoint End of G1, just before onset of the S phase (DNA replication) Yeast “start”; other eukaryotes “restriction point” The options for the cell at this point: • divide, delay division, or exit the cell cycle Cells can exit the cell cycle at this point into an arrested stage (G0) When this checkpoint is passed, cdk4 and cyclin D interact. This interaction results in phosphorylation of the retinoblastoma protein, which in turn allows activation of the transcription factor E2F. Active E2F promotes expression of the cyclin E gene. Cyclin E (protein) and cdk 2 interact to promote the G1 to S phase transition. (Figure available here.) p53 Mutations at the G1 Checkpoint • The gene encoding the p53 tumor suppressor protein is essential for stopping cells with damaged DNA from entering S phase. • Mutations in the p53 gene are found in more than 50% of human cancers. • Many mutations in p53 gene eliminate its ability to activate expression of target genes like the p21 gene. The p21 protein is a cyclin kinase inhibitor (CKI) that inhibits activity of the G1 cyclin-cdk complexes. Cell Cycle Checkpoints • DNA Replication Checkpoint (end of G2) Cell will not proceed with mitosis if DNA replication is not complete The way the cell senses this is not understood completely This checkpoint involves signals that block the activation of M phase cyclin-cdk complex (MPF) by inhibiting the activity of cdc25 protein phosphatase. Cells with mutations in this checkpoint pathway or cultured mammalian cells treated with caffeine will proceed through mitosis with unreplicated DNA. Cell Cycle Checkpoints • Growth checkpoints In budding yeasts, division produces a small daughter cell and a large mother cell. The daughter cell spends a longer time growing in G1 before it can divide again. There is a minimum size that must be reached before S phase can begin. In animal cells, external growth factors play a major role in providing signals about cell growth and differentiation and regulating the cell cycle. Cell Cycle Checkpoints • Spindle-attachment checkpoint Before anaphase (separation of chromosomes) there is a checkpoint to ensure the chromatids are correctly attached to the mitotic spindle The kinetochore (where the chromatids attach to the spindle) is the structure that is monitored Unattached kinetochores negatively regulate the activity of cdc20anaphase promoting complex (APC), and anaphase is delayed Cell Cycle Checkpoints • Exiting Mitosis Degradation of the M phase cyclin/cdk complex (aka MPF) is required to proceed with the final activities of mitosis (spindle disassembly and formation of the nuclear envelopes). This degradation is accomplished by ubiquitinylation of the complex. The cdc20-APC complex is responsible for signaling the degradation and exit from mitosis. Some Cell Cycle Summary Figures • A table listing some of the players in cell cycle regulation and where they act. • A schematic summary of the cell cycle regulators. Developmental Links • Because cell divisions have to start and stop at the right times during development, many developmental events are marked by changes in the cell cycles • Cell cycle regulation and regulation of developmental transitional events are often intertwined Early Embryonic Divisions in Xenopus • After fertilization, the Xenopus embryo goes through 12 rapid and synchronous cell cycles which are essentially alternating M and S phases (G1 and G2 are dramatically reduced). • These cell cycles are driven by maternally supplied proteins and mRNAs • Mid-blastula transition (MBT): At cycle 13 maternal transcripts are destroyed; zygotic transcription begins; cell cycles change to include extended G1 and G2 phases • How is this developmental transition accomplished? • The answer lies in miRNAs, which bind to sequences in the 3´ untranslated regions of maternal RNAs and direct their deadenylation, leading to their degradation.