Infrared Spectroscopy
Chemistry 243
Infrared spectral regions
cm
-1
μm 10
1
4
Hz
μm
cm
c cm /s
What is IR measuring?
Radiation not energetic enough to promote
electronic transitions, but rather vibrational
and rotational motions
Absorption results in an increase in amplitude of
the motion
Selection rule: Molecule (mode) must
undergo a net change in dipole moment to be
IR active
This determines whether a given mode absorbs in
the IR or not
Types of molecular vibrations
Stretches
and bends
Total number of
vibrational modes (not
all active):
3N-6
3N-5
for linear molecules
N
= total number of atoms
involved in motion
Skoog, Fig. 16-2
Harmonic oscillator, Hook’s
law and anharmonicity
Harmonic
oscillator
model
F ky
E
1
2
ky
2
Anharmonic
oscillator
model
in blue
Skoog, Fig. 16-3
A real vibrational transition acts like an anharmonic oscillator due to
coulombic repulsion at short distances, and dissociation at long
distances; different limit conditions than a harmonic oscillator
Vibrational frequencies: IR
(and Raman – more later)
For 2 atoms, vibration
m
1 k
2
frequency,
1/ 2
(in Hz)
k force constant
m
(wavenumbe
-1
rs in cm )
c
(N/m kg/sec
reduced mass (kg)
m
2
)
c speed of light (cm/sec)
m1 m 2
m1 m 2
Vibrations are quantized—can only assume discrete energies
If energy is absorbed in the IR or Raman region, that’s the E
Vibrational energy levels are equally spaced and transitions can
only occur if the vibrational quantum number changes by ± 1.
“Forbidden” overtones are observed at higher frequencies as
a result of anharmonicity (v = ± 2,3 observed at 2 and 3,
respectively)
Instrumentation for measuring
IR absorption
Dispersive spectrometers
Fourier transform spectrometers
Source deliberately has a broad emission spectrum
Use a grating monochromator to select wavelength
Wavelength scanned by rotating grating
Multiplexed measurement that has fewer optical
components
No gratings, slits, etc.
Most common; the Michelson interferometer for IR
Nondispersive photometers
Use filter (or absorbing gas as a filter) to select specific
probe wavelengths for a given atmospheric gas
Dispersive instruments for IR
(Dispersive = intentional use of many wavelengths)
Generally double-beam
dispersive instruments
Lessens demands on sources
and detectors
Double Beam in Time
Double beam geometry
compensates for drift issues
Low intensity sources and
low sensitivity detectors
Compensates for
environmental interferences
(such as water and CO2 in the
lab atmosphere)
Sample located before
monochromator
Skoog, Fig. 16-11
% Transmittance
Typical IR Spectrophotometer
Output
Skoog, Fig. 16-10
Why %T instead of Absorbance? Just tradition.
IR sources—low intensity
Inert solids heated between 1500-2200 oK
Nernst glower
Best for far-IR ( > 50 m)
Tungsten filament lamp
Resistively heated nichrome or rhodium wire—lower intensity but longer life
High pressure Hg arc lamp
Silicon carbide rod—better than Nernst for >5 m
Incandescent wire
Rare earth oxides that is resistively heated
Globar
Approximately blackbody, continuum peaked at ~2 m
Convenient for near-IR—often comes with UV-vis spectrometers
CO2 laser
Tunable through ~100 discrete lines between 900-1100 cm-1
Very high power (~2 orders better than thermal sources)
Valuable for light detection and ranging (LIDAR)
IR transducers—low sensitivity
Pyroelectric
Material that has strong temperature dependent polarization
IR absorption leads to heating that in turn causes a current
change in the external circuit connected to the capacitor
Fast response allows time-domain interferometric measurements
Photoconducting
Thin film of material that promotes valence electrons to
conduction band upon absorption of IR
PbS for near-IR @RT, MCT (HgCdTe) for mid- and far-IR @ 77oK
Thermal—limited by surrounding thermal noise
Thermocouples
Junction of wires that generate a potential difference with
temperature variation (30 ms response)
Thermistor (Bolometer)
Single material that has a resistance change as a function of
temperature (few ms response)
Result: Use FT spectrometers for
IR absorption
Typically a Michelson interferometer
Light throughput (Jaquinot) advantage
Reduced number of optical elements (no slits needed for
time data that will be transformed into frequency data)
leads to more transmitted light (greater S/N)
Wavelength accuracy (Connes) advantage
This is the approach that takes us from time domain
measurements to frequency domain results
Superior frequency data can be converted to
Multiplex (Fellgett) advantage
Entire spectrum taken simultaneously in ~ 1 second or less
Many more spectra can be acquired in a given time frame
and thus S/N increases as 𝑛
Advantages of FT
spectrometers
2-3 orders of magnitude higher throughput
Partially offset by lower sensitivity of fast-response detector
No stray light
High resolution (<0.1 cm-1)
Highly accurate and reproducible frequency determination
Rapid scanning—few seconds for good spectra
Good S/N ratios
Particularly in mid-IR (4000-200 cm-1)
Radiant Power
Measuring optical spectra in time
rather than frequency space
Skoog, Fig. 7-41
Measuring optical spectra in time
rather than frequency space
The time-domain signal contains the same
information as the frequency domain signal
Light throughput (Jaquinot) advantage
Wavelength accuracy (Connes) advantage
Multiplex (Fellgett) advantage
You can convert one to another, in either direction
P ( t ) k cos ( 2 1 t ) k cos ( 2 2 t )
k constant
t time
Measuring optical spectra in the time
domain but converting to frequency
domain
Measuring optical spectra in the time
domain but converting to frequency
domain
Here, w = frequency
Problem!!!
How can a signal fluctuating at 5.5 x 1013 Hz
be measured in time?
Would require a sub-picosecond response
time photodetector!
Michelson interferometer
Converts high frequency
optical signal (1012 - 1015 Hz)
to observable frequency
(otherwise too fast to observe
directly)
Radiation is split into two
beam paths of varying path
length.
Recombination gives
interference that is dependent
upon difference in path length
for a given optical frequency.
Path length is dynamically
changed to give a lower
frequency read-out.
could be called D,
but it’s called the mirror retardation
partially
reflective;
about 50/50
Fixed
focusing
mirror
Skoog, Fig. 7-43
Interferograms – the output of a Michelson
interferometer; typically for optical work
Monochromatic
Dual
Many clumped ’s
Observed
pattern
Detector
signal
versus d
Detector
signal FT
into frequency
spectrum
Skoog, Fig. 7-44
Advantages of Fourier transform
optical measurements
Light throughput (Jaquinot) advantage
Wavelength accuracy (Connes) advantage
Reduced number of optical elements (no slits needed for
time data that will be transformed into frequency data)
leads to more transmitted light (greater S/N)
Superior frequency data can be converted to
Multiplex (Fellgett) advantage
Entire spectrum taken simultaneously in ~ 1 second or less
Many more spectra can be acquired in a given time frame
and thus S/N increases as 𝑛
Advantage in IR spectroscopy, NMR, MS, MRI
If data is squeezed in time, it is stretched out in Hz
Gratings vs. Interferometers
Frequency vs. Time Domain Measurements
Gratings use
frequency or
Multiple wavelengths
require scanning or
array detectors
Typically no real-time
wavelength reference
Easier conceptual
operation
Multiplex
- disadvantage in IR
- advantage in UV
An interferometer
uses the time domain
Multiple wavelengths
inherent; FFT yields a
freq-sorted spectrum
Real-time wavelength
reference
Higher light throughput
at constant resolution
Multiplex
- advantage in IR
- disadvantage in UV
Shot and flicker noise, in contrast to detector noise, increase with radiant power
Detector noise is prevalent in IR, so interferometry is better. Not so much for UV-Vis
Single-beam FTIR
spectrometer
Skoog, Fig. 16-8
Double-beam FTIR
spectrometer
Why?
Skoog, Fig. 16-9
General comments on
analytical IR spectrometry
More challenging than UV-vis because there aren’t
many good solvents
Water is a problem
Useful for quantitative analysis
Mid-IR
Strong IR absorption and dissolves many window materials
CO2 is a problem—gives background signal
Near-IR
Need high concentrations with low pathlength cells
Mostly good for qualitative organic compound analysis
Far-IR
Useful for inorganic studies with low frequency vibrations
Qualitative Mid-IR
spectrometry
Organic chemistry
Skoog, Fig. 17-5
See Fig. 17-6 for more
Reflective IR spectrometry
Diffuse reflectance
Very useful for powdered samples
Peak positions (but not necessarily
intensities) same as absorption
spectra.
Attenuated total reflection (ATR)
Allows analysis of complex
samples and is not sensitive to
sample thickness
Spectra similar to standard
absorption with exceptions near
strong absorption bands
Skoog, Fig. 17-11 & 13
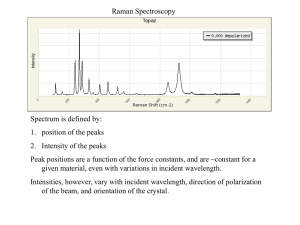
Raman scattering
Raman scattering discovered
by C.V. Raman in 1928—
received 1931 Nobel Prize for
physics
Excitation by a high intensity
source to a non-quantized
“virtual state”
Emission of a new photon at
energies plus or minus the
vibrational energy can be
measured
Emission at same wavelength
is Rayleigh scattering
Raman spectroscopy
Stokes and anti-Stokes lines
are observed.
Skoog, Fig. 18-1
Ratio of Stokes and anti-Stokes is
temperature dependent Why?
Very weak—best case is
0.001% of incident power
Similar information as in IR
spectra, but different selection
rules make techniques
complementary
Selection rule requires
change in polarizability
(induced dipole)
For molecules with center of
symmetry, same mode cannot
be Raman and IR active
Measurements can be made in
the visible spectral region
Emission Energies
This is a good thing!
For 2 atoms, vibration
m
1 k
2
frequency,
m
same as m
1/ 2
(in Hz)
k force constant
(N/m kg/sec
reduced mass (kg)
m1 m 2
m1 m 2
2
)
Raman instrumentation
Laser excitation required because high intensity is
needed to get measurable scattering
Spectra almost always collected at 90o to avoid
intense excitation light
Fiber optic grating and filterbased Raman spectrometer
BP is a bandpass filter and BR is a band-rejection filter (usually a notch
filter) used to remove Rayleigh scattering.
FT-Raman
Applications of Raman
spectroscopy
Inorganic species
Organic species
Can look at aqueous solutions
Metal-ligand bonds at 100-700 cm-1 hard to study with IR
absoprtion
Valuable for qualitative analyses for IR silent modes
Biological applications
Quantitative applications
Raman spectra are less crowded than IR.
Resonance and surface-enhanced
Raman spectroscopy (SERS)
Resonance Raman
Excitation near an
electronic transition can
enhance Raman signals
from symmetric vibrations
by 102-106
Surface-enhanced Raman
scattering (SERS)
When the scatterer is
located proximal to metallic
nanotructures that support
large electromagnetic fields,
Raman signals are
enhanced up to 1012
This has allowed single
molecule detection!!!
Van Duyne, et.al.; Anal. Chem. 2005, 77, 338A-346A.
Comparison of IR and Raman
Complementary techniques used
to probe vibrational energy levels
of molecules
For 2 atoms, vibration frequency, m
Raman scattering is incredibly
1/ 2
weak, but has the advantage of
1 k
(in Hz)
m
visible region operation
2
More applicable in aqueous
2
k force constant (N/m kg/sec )
(biological) environments
More material (cell) flexibility
m1 m 2
reduced mass (kg)
More sensitive detectors
m1 m 2
available
Raman rapidly becoming more
widespread as peripheral
technologies improve