Raman_Intensities
advertisement
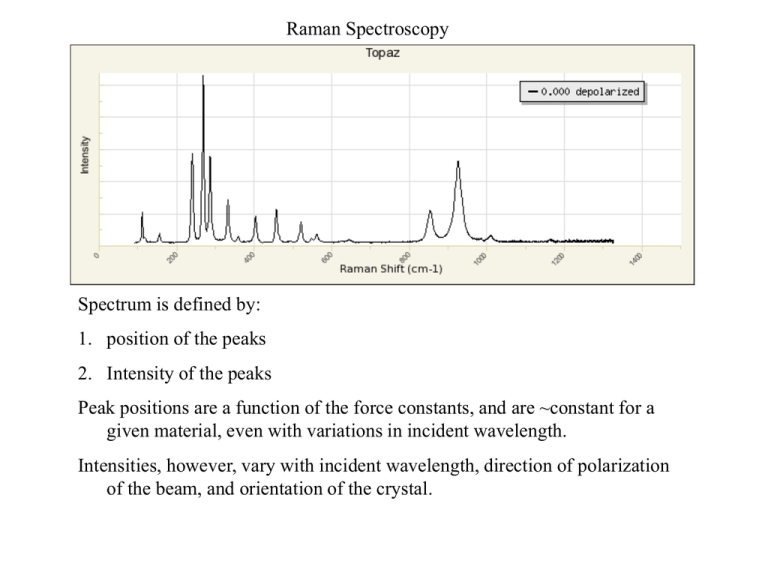
Raman Spectroscopy Spectrum is defined by: 1. position of the peaks 2. Intensity of the peaks Peak positions are a function of the force constants, and are ~constant for a given material, even with variations in incident wavelength. Intensities, however, vary with incident wavelength, direction of polarization of the beam, and orientation of the crystal. Laser parallel to c Laser parallel to a, polarized parallel to c at angle = 0 Intensities are difficult to compute •Arm waving explanation, you can get Raman intensity if the vibration of the atoms causes a change in the polarization of the electron density at the macro scale. Of course every vibration of an atom causes a change in the polarization of the electron density at the atomic scale. •Important case, if an atom is located on a point of inversion, then any vibration in one direction is associated with another vibration in the exact opposite, resulting in no change in macro scale polarization, and therefore, no peak intensity associated with the vibration of that atom. •Consequences, rocksalt and ccp elements have no Raman spectra because every atom is on a center of inversion. •Eg. Calcite, CaCO3. Ca is on a center of inversion, so Raman is only associated with CO3 vibrational modes. Calcite, peak at 280 cm-1, laser along a*, horizontal is // b, vertical is // c, rotate crystal in 5° increments Distance from center of plot is proportional to intensity. Vibrational mode is rocking of planar CO3 group about a* axis. Laser parallel to c •In this case, there is no variation in intensity for any of the different vibrational mode as the polarization of the beam is varied. •That is because we are shooting down an axis of 4-fold symmetry, so the optical ellipsoid is circular. All properties of a crystal show no variation as a function of polarization when examined along the axes of 3-, 4-, or 6-fold symmetry. •In particular, there is no change in the polarizability of the electron density in rutile when the beam is directed along the c-axis. Crystal structure of rutile, TiO2, looking along the a axis. Note that maximum in 450 cm-1 peak is when polarization direction is nearly parallel to the TiO bond Topaz, Al2SiO4F2, orthorhombic Let’s examine the Si-O bond stretching modes. There are 3 non-equivalent SiO bonds, R(SiO1) = 1.636 Å R(SiO2) = 1.648 Å R(SiO3) = 1.640 Å twice SiO3 SiO2 SiO1 b c Red = short Green = long Blue = medium x 2 Laser along a-axis, 0 = b-axis, 90 = c-axis b Red = short a Green = long Blue = medium x 2 Laser along c-axis, 0 = a-axis, 90 = b -axis c Red = short a Green = long Blue = medium x 2 Laser along b-axis, 0 = c-axis, 90 = a-axis In general, tetrahedral groups produce strong peaks, while octahedral groups do not. Question, why is this so?