Slides - IISER Pune
advertisement

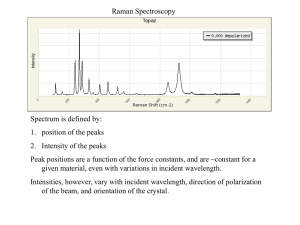
Introduction to Basics of Raman Spectroscopy Chandrabhas Narayana Chemistry and Physics of Materials Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur P.O., Bangalore 560064, India cbhas@jncasr.ac.in http://www.jncasr.ac.in/cbhas Lecture at MASTANI Summer School, IISER, Pune, June 30, 2014 to July 12, 2014 July 11, 2014 What happens when light falls on a material? Transmission Reflection Absorption Luminescence Elastic Scattering Inelastic Scattering Raman Spectroscopy 1 in 107 photons is scattered inelastically Scattered Excitation virtual state Rotational Raman Vibrational Raman Electronic Raman v” = 1 v” = 0 Infrared Raman (absorption) (scattering) Raman visible through unaided eye Raman, Fluorescence and IR Scattering Absorption and emission Absorption Concept of normal modes in a molecule • There are 3N possible movements in a molecule made of N atoms, each of which moving in one of three directions, x, y and z. – There are three transitional movements: all atoms in the molecule moving in x, y or z direction at the same time. – There are three rotational movements around x, y or z-axis • Linear molecules are exceptions because two axes that are perpendicular to the molecular axis are identical. – The rest of movements are vibrational movements • For linear molecules, 3N – 5 movements • For non-linear molecules, 3N – 6 movements – All vibrational movements of the sample can be described as linear combinations of vibrational normal modes. Vibrations in Molecules Sym. Stretching HCl n = 2991 cm-1 8086 cm-1 = 1 eV HF Asym. Stretching Sym. Bending n3 = 3939 cm-1 n2 = 1648 cm-1 H2O n1 = 3835 cm-1 n = 4139 cm-1 Asym. Bending NH3 n1 = 3505.7 cm-1 SF6 n1 = 774.55 cm-1 n3 = 3573.1 cm-1 n5 = 643.35 cm-1 n3 = 947.98 cm-1 n2 = 1022 cm-1 n2 = 615.02 cm-1 n4 = 1689.7 cm-1 n6 = 348.08 cm-1 n4 = 523.56 cm-1 Vibrational Spectroscopy DAB For a diatomic molecule (A-B), the bond between the two atoms can be approximated by a spring that restores the distance between A and B to its equilibrium value. The bond can be assigned a force constant, k (in Nm-1; the stronger the bond, the larger k) and the relationship between the frequency of the vibration, , is given by the relationship: 0 rAB k or, more typically 2cn k where , c is the speed of light, n is the frequency in “wave numbers” (cm-1) and is the reduced mass (in amu) of A and B given by the equation: m m re A B mA mB re = equilibrium distance between A and B DAB = energy required to dissociate into A and B atoms Vibrational Spectroscopy 2cn k can be rearranged to solve for k (in N/m): k 5.89 10 5 n2 Molecule n (cm-1) k (N/m) (amu) HF 3962 878 19/20 HCl 2886 477 35/36 or 37/38 HBr 2558 390 79/80 or 81/82 HI 2230 290 127/128 Cl2 557 320 17.5 Br2 321 246 39.5 CO 2143 1855 6.9 NO 1876 1548 7.5 2331 2240 7 N2 For a vibration to be active (observable) in an infrared (IR) spectrum, the vibration must change the dipole moment of the molecule. (the vibrations for Cl2, Br2, and N2 will not be observed in an IR experiment) For a vibration to be active in a Raman spectrum, the vibration must change the polarizability of the molecule. Classical Picture of Raman Induced Polarization Polarizability Stokes Raman Anti-Stokes Raman Mutually exclusive principle Raman Scattering Selection rule: v = ±1 Overtones: v = ±2, ±3, … z (t ) zzequil Emax cos 2n 0t 1 d zz rmax Emax cos 2 (n 0 n vib )t 2 dr 1 d zz rmax Emax cos 2 (n 0 n vib )t 2 dr Must also have a change in polarizability Classical Description does not suggest any difference between Stokes and Anti-Stokes intensities N1 e N0 www.andor.com hn vib kT Are you getting the concept? Calculate the ratio of Anti-Stokes to Stokes scattering intensity when T = 300 K and the vibrational frequency is 1440 cm-1. h = 6.63 x 10-34 Js k = 1.38 x 10-23 J/K N1 e N0 hn vib kT ~ 0.5 Energy diagram and Quantum picture Virtual states Raman cross section S photon <eg,p2|Her|p2,eb> <eb,p2|Hep|p1,ea> <ea,p1|Her|p1,eg> |Es-Eb|x|Ei-Ea| a,b ex g Electronic states Vibrational states If Ei = Ea or Es = Eb We have Resonance Raman effect • • • • • Intensity of Normal Raman Peaks The intensity or power of a normal Raman peak depends in a complex way upon the polarizability of the molecule, the intensity of the source, and the concentration of the active group. The power of Raman emission increases with the fourth power of the frequency of the source; - photodecomposition is a problem. Raman intensities are usually directly proportional to the concentration of the active species. Raman Depolarization Ratios Polarization is a property of a beam of radiation and describes the plane in which the radiation vibrates. Raman spectra are excited by plane-polarized radiation. The scattered radiation is found to be polarized to various degrees depending upon the type of vibration responsible for the scattering. Raman Depolarization Ratios The depolarization ratio p is defined as I p I Experimentally, the depolarization ratio may be obtained by inserting a polarizer between the sample and the monochromator. The depolarization ratio is dependent upon the symmetry of the vibrations responsible for scattering. Raman Depolarization Ratios Polarized band: p = < 0.76 for totally symmetric modes (A1g) Depolarized band: p = 0.76 for B1g and B2g nonsymmetrical vibrational modes Anomalously polarized band: p = > 0.76 for A2g vibrational modes Raman spectra of CCl4 Isotope effect Cl has two isotopes 35Cl and 37Cl Relative abundance is 3:1 CCl4 Spectra • 461.5 cm-1 is due to 35Cl4C • 458.4 cm-1 is due to 35Cl337ClC • 455.1 cm-1 is due to 35Cl237Cl2C • What are the two question marks? • Why are these bands weak? Raman Spectra of Methanol and Ethanol CH stretching 20000 Raman Intensity (arbitrary unit) CCO stretching CH3 and CH2 deformation 15000 OH stretching 10000 CO stretching CH3 deformation 5000 0 500 1000 1500 2000 2500 3000 3500 Raman Shift (cm-1) CASR Significant identification of alcohols which differ just in one CH2-g Peak position – Chemical identity – Similar Structures CASR 3,4-Methylenedioxymethamphetamine (MDMA) Methamphetamine ecstasy Raman Intensity (arbitrary unit) 500 1000 1500 2000 Raman Shift (cm-1) 2500 3000 3500 The Mass Effect on Raman Spectra Significant identification of salts (SO42-) which differ just in the metal ion employed 1200 1000 Intensity (counts/s) 800 600 400 1000 200 0 Wavenumber (cm-1) Intensity (counts/s) 800 600 Mg - SO4 Na2 - SO4 400 200 Wavenumber (cm-1) CASR CASR Peak positions – Chemical identity Diasteromers Ephedrine Pseudoephedrine Raman Intensity (arbitrary unit) 500 1000 1500 2000 Raman Shift (cm-1) 2500 3000 3500 Peak Position – Crystal Phases – Polymorphs Both Anatase and Rutile are TiO2 but of different polymorphic forms - identical chemical composition, different crystalline structures. 2 400 2 200 2 000 Rutile 1 800 Intensity (cnt) 1 600 1 400 1 200 1 000 800 600 400 200 200 400 600 800 1 000 1 200 Raman Shift ( cm-1) Anatase 1 400 CASR Peak Shift – Stress and Strain Larnite (b – Ca2SiO4) inclusion in Diamond Nasdala, L., Harris, J.W. & Hofmeister, W. (2007): Micro-spectroscopy of diamond. Asia Oceania Geosciences Society, 4th Annual Meeting, Bangkok, Thailand, August, 2007. Nasdala, L., Raman barometry of mineral inclusions in diamond crystals. Mitt. Österr. Miner. Ges. 149 (2004) CASR Bandwidth – Crystallinity – Structural order/disorder Raman spectra of zircon, showing typical amorphous (blue) and crystalline (red) spectra. Intensity – Concentration CASR 1341.0 4-Nitrophenol dissolved in CH2Cl2 3 500 Intensity (cnt/sec) 3 000 4-Nitrophenol in CH2Cl2_0,1 M 4-Nitrophenol in CH2Cl2_0,01 M 4-Nitrophenol in CH2Cl2_0,001 M 2 500 2 000 1 500 1 000 500 0 1 200 1 400 Raman Shift (cm -1 ) 1 600 CASR Raman technique – what requirements are needed? Requirements for Raman technique to determine peak position, peak shift, bandwidth and intensity - Laser Excitation - Reduction of stray light - Collecting Optics - Spectral resolution and spectral coverage - Spatial resolution and confocality - Sensitivity: subject to detector - Light flux: subject to dispersion CASR What do we need to make a Raman measurement ? Detector •Monochromatic typically a laser Light source (A way of focusing the laser onto the sample and then collecting the Raman scatter.) •Sampling optics Grating •Rejection filter (A way of removing the scattered light that is not shifted( changed in colour). Filter Laser Sample •Spectrometer and detector (often a single grating spectrometer and CCD detector.) Demonstration of the very high CASR spectral resolution obtained in the triple additive mode Rotation-Vibration Spectrum of O2 Intensity (a.u.) 8000 Triple additive mode Slit widths= 30 m 6000 4000 Triple subtractive mode . Slit=30 m 2000 1520 1540 1560 Wavenumber (cm-1) 1580 CASR Laser excitation – Laser Selection to avoid fluorescence Laser wavelength, 3 Laser wavelength, 3 Laser wavelength, 1 Fluorescence Raman shift, 1-1+ Laser wavelength: 3 < 2 < 1 Raman shift, 3-1+ Laser excitation – Laser selection to avoid fluorescence CASR 60 000 Green spectrum: 532 nm laser Red spectrum: 633 nm laser Dark red spectrum: 785 nm laser 50 000 Intensity (cnt) 40 000 30 000 20 000 10 000 60 000 0 50 000 800 Wave le ngth (nm) 1 000 40 000 Intensity (cnt) 600 30 000 Fluorescence is wavelength dependent Ordinary Raman is wavelength independent 20 000 10 000 0 1 000 2 000 Raman Shift (cm-1) 3 000 Laser excitation – Laser selection to CASR avoid fluorescence Commercial Hand Cream x10 3 45 40 35 Intensity (cnt) 30 25 20 15 10 5 500 1 000 1 500 785 nm – 633 nm – 473 nm 2 000 Raman Shift (cm-1 ) 2 500 3 000 3 500 Reduction of Fluorescence CASR Laser excitation – laser radiation power Laser wavelength: 473 nm Laser power at sample: 25.5 mW Laser wavelength: 633 nm Laser power at sample: 12.6 mW Objective N.A. Objective N.A. Laser spot size (µm) Radiatio n power (kW/cm2) 100× 0.90 0.64 ~7900 100× 0.90 0.85 ~2200 50× 0.75 0.77 ~5400 50× 0.75 1.03 ~1500 10× 0.25 2.31 ~600 10× 0.25 3.09 ~200 Laser Radiation spot size power (µm) (kW/cm2) CASR Laser excitation – laser radiation power • Keep in mind: the usage of high numerical objective lenses causes a very small spot size of the laser which results in a high power density • To avoid sample burning radiation power has to be adapted INDIVIDUALY to the sample Collection solid angle Large for high N.A. lens Small for low N.A. lens Low N.A. lens Working distance θ High N.A. lens Sampling volume Small for high N.A. lens Large for low N.A. lens Laser spot size Small for high N.A. lens Large for low N.A. lens NA = n · sin (Q) n: refraction index Q: aperture angle θ Working distance Collecting Optics CASR CASR Collecting Optics – Overview on common objectives Objective N.A. Working distance [mm] x100 0.90 0.21 x50 0.75 0.38 x10 0.25 10.6 x100 LWD 0.80 3.4 x50 LWD 0.50 10.6 Collecting Optics – what objective should be used? CASR A distinction between opaque and transparent samples has to be made For opaque samples, high N.A. lens works better because there is almost no penetration of the laser into the sample. High N.A. lens enables - High laser power density (mW/m3) increases sensitivity - Wide collection solid angle increases sensitivity Example for an opaque sample: Silicon wafer 100 % 30 000 Silicon x100 – NA = 0.9 – 31.350 C/s x50 – NA = 0.75 - 21.995 C/s x10 – NA = 0.25 - 1.462 C/s 25 000 70 % 20 000 Intensity (cnt/sec) Y (µm) -20 15 000 0 10 000 10 µm 20 5 000 5% 0 0 X (µm) 460 480 500 520 540 Raman Shif t (cm-1) 560 580 600 620 Collecting Optics – what objective should be used? CASR A distinction between opaque and transparent samples has to be made For transparent samples, low N.A. lens works better because of penetration of the laser into the sample. Low N.A. lens enables - Large sampling volume increases sensitivity x10 3 14 12 Sample: Cyclohexane Instrument: ARAMIS Red: x100LWD, 7,000 cts/s Blue: Macro lens, 14,500 cts/s cyclo_100xLWD cyclo_macro 100 % Intensity (cnt) 10 8 48 % 6 4 2 0 740 760 780 800 820 Raman Shift (cm-1 ) 840 860 880 Spectral resolution and spectral coverage CASR Schematic diagram of a Czerny-Turner spectrograph Slit Collimating mirror Grating Detector Focusing mirror Focal Length CASR Spectral resolution and spectral coverage • Spectral resolution is a function of 1. dispersion, 2. widths of entrance slit and 3. pixel size of the CCD • Dispersion is the relation between refraction of light according to the wavelength of light • Dispersion is a function of the 1. focal length of spectrograph the 2. groove density of the grating and 3. the excitation wavelength • In general, long focal length and high groove density grating offer high spectral resolution. CCD Detector Short focal length Same grating Same excitation wavelength Long focal length CCD Detector CASR Dispersion as a function of the focal length CASR Dispersion as a function of the focal length vis-a vis wavelength Dispersion in cm-1 / pixel 1800 gr/mm Grating LabRAM (F = 300 mm) LabRAM HR (F = 800 mm) Dispersion as a function of excitation wavelength CASR Horizontal lines indicate a relative Raman Shift of 3800 cm -1 244 - 269 nm (25 nm) 325 - 371 nm (46 nm) 457 - 553 nm (96 nm) 488 - 599 nm (111 nm) 514 - 639 nm (125 nm) 532 - 667 nm (135 nm) 633 - 833 nm (200 nm) 785 - 1119 nm (334 nm) 830 - 1210 nm (380 nm) 1064 - 1768 nm (704 nm) 200 400 600 800 1000 1200 1400 1600 1800 Wavelength [nm] Short wavelength CCD Detector CCD Detector Same focal length Same grating Long wavelength CASR Spectral coverage - dependence from excitation wavelength Length of CCD Chip x10 3 473 nm – 633 nm – 785 nm 22 20 18 16 Intensity (cnt/sec) Same focal length Same grating Length of CCD Chip 14 12 10 Length of CCD Chip Relative Raman shift of 3100 cm-1 8 corresponds to 81 nm 6 Relative Raman shift of 3100 cm-1 corresponds to 154 nm 4 2 Relative Raman shift of 3100 cm-1 corresponds to 252 nm 0 500 600 700 800 Wavelength (nm) 900 1 000 Low density groove grating High density groove grating Same focal length Same excitation wavelength CCD Detector Dispersion as a function of groove density CCD Detector CASR CASR Spectral resolution as a function slit width Slit Slit Slit One of parameters that determines the spectral resolution is the entrance slit width. The narrower the slit, the narrower the FWHM (full width at half maximum), and higher the spectral resolution. When recording a line whose natural width is smaller than the monochromator’s resolution, the measured width will reflect the spectrograph’s resolution. Spectral resolution as a function of pixel CASR size Detector Intensity Detector 600 650 Raman Shift (cm -1) 700 • Because a CCD detector is made of very small pixels, each pixel serves as an exit slit (pixel size = exit slit width) • For the same size CCDs, the CCD with a larger number of smaller pixels produces a larger number of spectral points closer to each other increasing the limiting spectral resolution and the sampling frequency • 26 m pixel vs. 52 m pixel (simulation) Choice of Laser for Raman The choice of laser will influence different parameters: • Signal Intensity: (1/)4 rule applies to Raman intensity. • Probing volume: spot size material penetration depth. and • Fluorescence: may overflow Raman signal. 100 Silicium Penetration depth (µm) CASR 10 1 0,1 0,01 • Enhancement: some bounds only react strongly at a certain wavelength. • Coverage range and Resolution: grating dispersion varies along the spectral range. 0,001 200 300 400 500 600 700 Wavelength (nm) 800 900 CASR Spatial resolution: penetration depth Penetration depth in Silicon 244 nm 325 nm 0 2 4 6 Wavelength [nm] 244 nm 8 325 nm 457 nm 488 nm 514 nm 633 nm 0 10 500 Depth penetration [nm] 12 1000 1500 2000 2500 3000 General: The larger the excitation wavelength, the deeper the penetration. The exact values depend on material. Spatial resolution: penetration depth CASR 325 nm Intensity [a.u.] Strained Si of top layer 325 nm Si of SiGe layer ~nm ~nm 488 nm 785 nm Strained silicon layer Uniform layer of SiGe ~µm 488 nm Gradient SiGe layer Si of silicon substrate 400 450 500 550 785 nm Pure Si substrate Raman shift (cm-1) The higher the excitation wavelength, the deeper the penetration. Spatial resolution: penetration depth CASR EXAMPLE 325nm laser results The strain is not homogenous. A characteristic, cross-hatch pattern is observed. CASR Spatial resolution and spot size Laser spot size D is defined by the Rayleigh criterion: excitation wavelength () D = 1.22 / NA objective numerical aperture (NA) With NA=n sinα Spatial resolution is half of the laser spot diameter Confocality and Spatial Resolution CASR Nearly closed confocal apertur Sample P P P P' P' P' Image P ' of laser focus P matches exactly the confocal hole. Length of Laser Focus Axial resolution of a Confocal Raman Microprobe CASR Confocality and spatial Resolution Wide Hole Laser focus waist diameter Depth of laser focus (d.o.f) Sampling Volume Narrow Hole Confocal z-scan against silicon with different hole apertures exc = 633 nm CASR Narrow Hole: Collecting Raman radiation that originates only from within a diffraction limited laser focal volume with a dimension of: Focus waist diameter ~ 1.22 / NA Depth of laser focus ~ 4 / (NA)2 Example of application using the confocality principle : depth profile on a multilayer polymer sample 5000 Polyethylene nylon Polyethylene 75 m Intensity (a.u.) x 4000 3000 2000 1000 z 0 1000 1200 1400 1600 Wavenumber (cm-1) 3000 Intensity (a.u.) 2500 2000 1500 1000 500 0 CASR 1000 1200 1400 Wavenumber (cm-1) 1600 Thank you for your attention! Symmetry – Identity (E) Identity operation (E) This is a very important symmetry operation which is where the molecule is rotated by 360º. In otherwords a full rotation or doing nothing at all. This appears in all molecules!!! Symmetry – Rotation (Cn) Rotations (Cn) These are rotations about the axes of symmetry. n denotes 360º divided by the number for the rotation. C2 = 180º C3 = 120º C4 = 90º C5 = 72º C6 = 60º Symmetry – Reflections (s) Reflections (sh, sd and sv) These are reflections in a symmetry planes (x, y and z). sh - Horizontal Plane (y) perpendicular to the highest rotation axis sv - Vertical Plane (z) parallel to the highest rotation axis sd - Diagonal (dihedral) Plane (x) the Diagonal Plane that bisects two axes Symmetry – Inversion (i) Inversion centre (i) These are where the molecule can be inverted through the centre of the molecule (atom or space). Symmetry – Improper Rotation (Sn) Improper rotations (Sn) These are rotations about the axes of symmetry followed by reflections. Vibrational Spectroscopy For polyatomic molecules, the situation is more complicated because there are more possible types of motion. Each set of possible atomic motions is known as a mode. There are a total of 3N possible motions for a molecule containing N atoms because each atom can move in one of the three orthogonal directions (i.e. in the x, y, or z direction). A mode in which all the atoms are moving in the same direction is called a translational mode because it is equivalent to moving the molecule - there are three translational modes for any molecule. A mode in which the atoms move to rotate (change the orientation) the molecule called a rotational mode - there are three rotational modes for any non-linear molecule and only two for linear molecules. Translation al modes Rotational modes The other 3N-6 modes (or 3N-5 modes for a linear molecule) for a molecule correspond to vibrations that we might be able to observe experimentally. We must use symmetry to figure out what how many signals we expect to see and what atomic motions contribute to the particular vibrational modes. Vibrational Spectroscopy and Symmetry We must use character tables to determine how many signals we will see in a vibrational spectrum (IR or Raman) of a molecule. This process is done a few easy steps that are similar to those used to determine the bonding in molecules. 1. Determine the point group of the molecule. 2. Determine the Reducible Representation, tot, for all possible motions of the atoms in the molecule. 3. Identify the Irreducible Representation that provides the Reducible Representation. 4. Identify the representations corresponding to translation (3) and rotation (2 if linear, 3 otherwise) of the molecule. Those that are left correspond to the vibrational modes of the molecule. 5. Determine which of the vibrational modes will be visible in an IR or Raman experiment. Finding the Point Group Vibrational Spectroscopy and Symmetry Example, the vibrational modes in water. The point group is C2v so we must use the appropriate character table for the reducible representation of all possible atomic motions, tot. To determine tot we have to determine how each symmetry operation affects the displacement of each atom the molecule – this is done by placing vectors parallel to the x, y and z axes on each atom and applying the symmetry operations. As with the bonds in the previous examples, if an atom changes position, each of its vectors is given a value of 0; if an atom stays in the same place, we have to determine the effect of the symmetry operation of the signs of all three vectors. The sum for the vectors on all atoms is placed into the reducible representation. O H H H O top view H Make a drawing of the molecule and add in vectors on each of the atoms. Make the vectors point in the same direction as the x (shown in blue), the y (shown in black) and the z (shown in red) axes. We will treat all vectors at the same time when we are analyzing for molecular motions. Vibrational Spectroscopy and Symmetry Example, the vibrational modes in water. z O The E operation leaves everything where it is so all nine vectors stay in the same place and the character is 9. H H The C2 operation moves both H atoms so we can ignore the vectors on those atoms, but we have to look at the vectors on the oxygen atom, because it is still in the same place. The vector in the z direction does not change (+1) but the vectors in the x, and y directions are reversed (-1 and -1) so the character for C2 is -1. H C2V E C2 sv (xz) s’v (yz) tot 9 -1 3 1 O H x C2 H The sv (xz) operation leaves each atom where it was so we H have to look at the vectors on each atom. The vectors in the z and x directions do not move (+3 and +3) but the vectors in the y direction are reversed (-3) so the character is 3. The s’v (yz) operation moves both H atoms so we can ignore the vectors on those atoms, but we have to look at the vectors on the oxygen atom, because it is still in the same place. The vectors in the z and y directions do not move (+1 and +1) but the vectors in the x direction is reversed (-1) so the character is 1. y O O H H sv (xz) H H O O H H s’v (yz) H O H Vibrational Spectroscopy and Symmetry C2V E C2 sv (xz) s’v (yz) tot 9 -1 3 1 C2V E C2 sv (xz) s’v (yz) A1 1 1 1 1 z x2,y2,z2 A2 1 1 -1 -1 Rz xy B1 1 -1 1 -1 x, Ry xz B2 1 -1 -1 1 y, Rx yz From the tot and the character table, we can figure out the number and types of modes using the same equation that we used for bonding: nX 1 # of operations in class) (character of RR) character of X) order This gives: nA1 1 1)9)1) 1)1)1) 1)3)1) 1)1)1) 4 nA 2 1 1 1)9)1) 1)1)1) 1)3)1) 1)1)1) 1)9)1) 1)1)1) 1)3)1) 1)1)1) nB2 4 4 nB1 1 1)9)1) 1)1)1) 1)3)1) 1)1)1) 4 Which gives: 3 A1’s, 1 A2, 3 B1’s and 2 B2’s or a total of 9 modes, which is what we needed to find because water has three atoms so 3N = 3(3) =9. Vibrational Spectroscopy and Symmetry Now that we have found that the irreducible representation for tot is (3A1 + A2 + 3B1+ 2B2), the next step is to identify the translational and rotational modes - this can be done by reading them off the character table! The three translational modes have the symmetry of the functions x, y, and z (B1, B2, A1) and the three rotational modes have the symmetry of the functions Rx, Ry and Rz (B2, B1, A2). Translation Rotational al modes modes C2V E C2 sv (xz) s’v (yz) A1 1 1 1 1 z x2,y2,z2 A2 1 1 -1 -1 Rz xy B1 1 -1 1 -1 x, Ry xz B2 1 -1 -1 1 y, Rx yz The other three modes (3(3)-6 = 3) that are left over for water (2A1 + B1) are the vibrational modes that we might be able to observe experimentally. Next we have to figure out if we should expect to see these modes in an IR or Raman vibrational spectrum. Vibrational Spectroscopy and Symmetry Remember that for a vibration to be observable in an IR spectrum, the vibration must change the dipole moment of the molecule. In the character table, representations that change the dipole of the molecule are those that have the same symmetry as translations. Since the irreducible representation of the vibrational modes is (2A1 + B1) all three vibrations for water will be IR active (in red) and we expect to see three signals in the spectrum. The three vibrational modes for water. Each mode is listed with a n (Greek letter ‘nu’) and a subscript and the energy of the vibration is given in parentheses. n1 is called the “symmetric stretch”, n3 is called the “anti-symmetric stretch” and n2 is called the “symmetric bend”. For a vibration to be active in a Raman spectrum, the vibration must change the polarizability of the molecule. In the character table, representations that change the polarizability of the molecule are those that have the same symmetry as the second order functions (with squared and multiplied variables). Thus all three modes will also be Raman active (in blue) and we will see three signals in the Raman spectrum. C2V E C2 sv (xz) s’v (yz) A1 1 1 1 1 z x2,y2,z2 A2 1 1 -1 -1 Rz xy B1 1 -1 1 -1 x, Ry xz B2 1 -1 -1 1 y, Rx yz The Geometry of the Sulfur Dioxide Molecule Cs structure: 3 normal modes, all having A' symmetry The Cs structure should have 3 IR active fundamental transitions. These three fundamental transitions also should be Raman active. We would expect to observe three strong peaks in the IR and three strong peaks in the Raman at the same frequency as in the IR. All of the Raman lines would be polarized because they are totally symmetric (A' symmetry). C2v structure: 3 normal modes, two with A1 symmetry, one with B2 The C2v structure should have 3 IR active fundamental transitions. These three fundamental transitions also should be Raman active.We would expect to observe three strong peaks in the IR and three strong peaks in the Raman at the same frequency as in the IR. Two of the Raman lines are totally symmetric (A1 symmetry) and would be polarized. One Raman line would be depolarized. The Dooh structure should have two IR active fundamental transitions. It will have one Raman active fundamental transition at a different frequency than either of the IR peaks.. The Raman line will be polarized. Experimental Observation The experimental infrared and Raman bands of liquid and gaseous sulfur dioxide have been reported in a book by Herzberg 7 . Only the strong bands corresponding to fundamental transitions are shown below. The polarized Raman bands are in red. Fundamental 2 1 3 IR (cm-1) 519 1151 1336 Raman (cm-1) 524 1151 1336 Conclusion The existence of three experimental bands in the IR and Raman corresponding to fundamental transitions weighs strongly against the symmetrical linear (Dooh) structure. We usually do not expect more strong bands to exist than are predicted by symmetry. Group theory predicts that both bent structures would have three fundamental transitions that are active in both the IR and Raman. However all three of the Raman lines would be polarized if the structure were unsymmetrical (Cs symmetry). The fact that one Raman line is depolarized indicates that the structure must be bent and symmetrical (C2v symmetry). The sulfur dioxide molecule has C2v symmetry. Problems with Raman: a)Very Weak – for every 106 photons only 1 photon Raman a)Resonant Raman not feasible with every sample. b)Absorption a better process than scattering International and National Patent (2007), G.V. Pavan Kumar et al Current Science (2007) 93, 778. Micro–Raman setup Raman Spectrometers Optical fiber CCD Monochromator Focusing lens Edge filter Camera Computer Dichroic Mirror LASER Objective lens Stage