The Shell Model and PES
advertisement

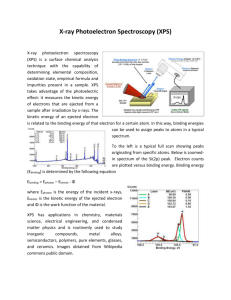
The Shell Model And Photoelectron Spectroscopy and the Structure of Atoms Ch 7 part 1.5 A little history Einstein earned the Noble Prize for his work on the Photoelectric effect. The photoelectric effect is when electrons are emitted from a solid liquid or gas when they absorb energy in the form of light. This has been determined experimentally by shining light on metal. Photoelectric Effect By shining a light on metal, one may create a current Photoelectric effect Increasing the intensity of the light increased the number of photoelectrons, but not their maximum kinetic energy Red light will not cause the ejection of electrons, no matter what the intensity! A weak violet light will eject only a few electrons, but their maximum kinetic energies are greater than those for intense light of longer wavelengths! Ephoton = hv Photoelectron Spectroscopy PES provides a useful means to extract information on atomic structure using spectroscopic data. By looking at the data chart one may determine the structure of the atom. How? The chart gives evidence for the shell model. Photoelectron Spectroscopy (PES) Do all electrons in a given shell have the same energy? To answer this a technique using radiation on a neutral atom in the gas phase called Photoelectron Spectroscopy is used. The technique shines radiation (UV or Xray) on an atom to excite it to the point that it will eject an electron from its shell and form a cation. Shell Model In the shell model, we see the ionization energy is decreased the farther the electron is from the nucleus. By the way of reasoning, it takes more energy to remove an electron from n=2 than n=3. Photoelectron Spectroscopy (PES) The energy of the photon used is higher than the ionization energy. The excess energy is converted to the kinetic energy on the ejected electron. The energy required to remove the electron is equal to the difference between the energy absorbed by the atom (hv) and the kinetic energy of the ejected electron IE = hv - KE Photoelectron Spectroscopy (PES) This technique allows the researcher to remove one electron from any shell. Therefore, One may remove electrons from the inner core without moving the outer electrons. The apparatus Reading a PES spectra On the PES spectra, energy increases as one moves to the left. The height refers to the number of electrons. Demostration http://www.chem.arizona.edu/chemt/Flas h/photoelectron.html