X-ray Photoelectron Spectroscopy (XPS)
advertisement
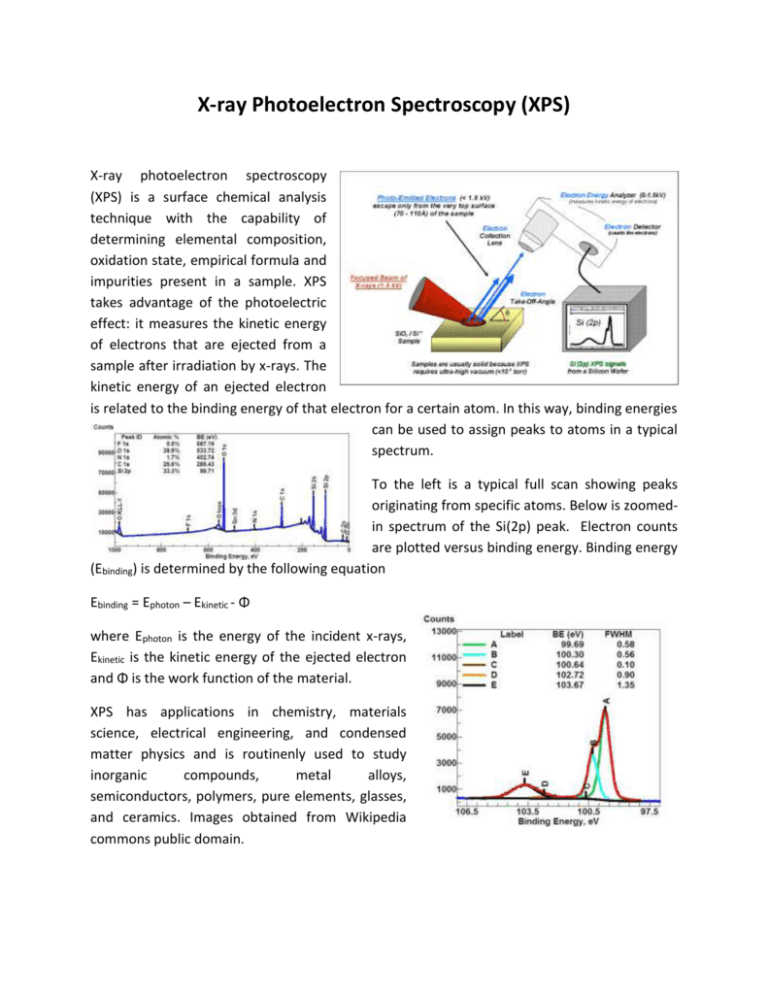
X-ray Photoelectron Spectroscopy (XPS) X-ray photoelectron spectroscopy (XPS) is a surface chemical analysis technique with the capability of determining elemental composition, oxidation state, empirical formula and impurities present in a sample. XPS takes advantage of the photoelectric effect: it measures the kinetic energy of electrons that are ejected from a sample after irradiation by x-rays. The kinetic energy of an ejected electron is related to the binding energy of that electron for a certain atom. In this way, binding energies can be used to assign peaks to atoms in a typical spectrum. To the left is a typical full scan showing peaks originating from specific atoms. Below is zoomedin spectrum of the Si(2p) peak. Electron counts are plotted versus binding energy. Binding energy (Ebinding) is determined by the following equation Ebinding = Ephoton – Ekinetic - Φ where Ephoton is the energy of the incident x-rays, Ekinetic is the kinetic energy of the ejected electron and Φ is the work function of the material. XPS has applications in chemistry, materials science, electrical engineering, and condensed matter physics and is routinenly used to study inorganic compounds, metal alloys, semiconductors, polymers, pure elements, glasses, and ceramics. Images obtained from Wikipedia commons public domain.











