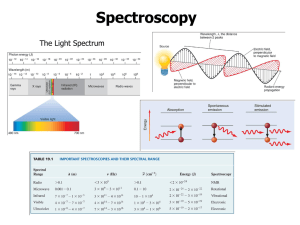
Photoelectron Spectroscopy
advertisement

Photoelectron Spectroscopy Drill • Use your own device to look up the vocab terms to help you answer this question. Answer What is Spectroscopy? • Spectroscopy pertains to the dispersion of an object's light into its component colors (i.e. energies). Definition • Photoelectron spectroscopy (PES) is a technique used for determining the ionization potentials of molecules. Question • Do all of the electrons in a given shell have the same energy? • This question can be answered by using PES to measure the energy required to remove an electron from a neutral atom in the gas phase to form a positively charged ion. What PES Can Do • PES can remove electrons from any shell in the atom. (valence or core electron) • Only a single electron is removed from a given atom, but that electron can come from any energy level. • PES allows us to measure the energy needed to remove any electron in an atom. PES Tutorials • http://www.sophia.org/tutorials/apchemistry-pes • or • http://youtu.be/tpNbBV7Hk6k What Happens During PES… • PES shines radiation on a sample. The radiation will have enough energy to excite an atom to the point that an electron can be ejected from any shell of the atom to form a positively charged ion. • The K.E. of the ejected electron is measured, and the energy required to remove the electron from the atom is calculated from the difference between the energy of the photon (hv) and the K.E. of the photoelectron. Spencer, p. 88-89 What Happens During PES… • A PES experiment begins with the absorption of a high-energy UV or X-ray photon that carries more energy than the I.E. of the atom. • The excess energy is carried off by the electron ejected from the atom in the form a K.E. • The energy of the photon (hv) absorbed by the atom is therefore equal to the sum of the I.E. of the atoms and the K.E. of the electron that is ejected from the atom. hv = I.E. + K.E. Spencer, p. 88-89 Calculation Kinetic energy of the photoelectron ejected when the radiation is absorbed Ionization Energy K.E. = hv - B.E. Energy of the radiation absorbed by the atom I.E. can also be called Binding Energy. This is the energy required to either separate an electron from an atom or to separate the protons and neutrons of an atomic nucleus. Equipment Diagram http://chemwiki.ucdavis.edu/Physical_Chemistry/Spectroscopy/Photoelectron_Spectroscopy/Photoelectron_Spectroscopy%3A_Application#Detection_.26_Spectra Detection • Detection relies on the ability of the instrument to measure energy and photoelectron output. One type of energy measured is the binding energy, which is calculated through the following equation: where: • KE = hν – BE Ke = Kinetic energy, this is measured hv = Photon energy from the radiation source, this is controlled by the source BE= Binding energy, this is the unknown of interest and can be calculated from the other variables http://chemwiki.ucdavis.edu/Physical_Chemistry/Spectroscopy/Photoelectron_Spectroscopy/Photoelectron_Spectroscopy%3A_Application#Detection_.26_Spectra Worksheet • Distribute the Photoelectron Spectroscopy WS to use for discussion. Spectral Output • The spectrum produced from a PES experiment has peaks that correspond to the ionization potentials of the molecule. These also correspond to the orbital energies. http://chemwiki.ucdavis.edu/Physical_Chemistry/Spectroscopy/Photoelectron_Spectroscopy/Photoelectron_Spectroscopy%3A_Application#Detection_.26_Spectra PES Data • Data from PES experiments are obtained as peaks in a spectrum that plots the intensity of the observed signal on the vertical axis versus the energy needed to eject an electron (IE) on the horizontal axis. Spencer, p. 89 PES Data • The spectrum is plotted so that energy increases from left to right on the horizontal axis. • The height of the peak in the PES spectra is directly proportional to the number of electrons of equivalent energy ejected during the experiment. Spencer, p. -89-91 PES Data • If you see two peaks that have a relative height ratio of 2:1, it may be concluded that one of the energy levels (from which electrons are removed) contains twice as many electrons as the other. Spencer, p. -89-91 Spectra • The following slides contain spectra generated from the following website: • http://www.chem.arizona.edu/chemt/Flash/p hotoelectron.html • http://www.pes.arizona.edu/aboutPES.htm • http://www.sisweb.com/mstools/isotope.htm Reading the Spectra • The data is graphed as spectrum of photoelectron counts versus kinetic energy Hydrogen has only one electron, therefore only one peak is shown. Notice that the peak for He is located farther to the right of the peak for H and the height of the peak is approximately twice the height of H. (There is only one peak because He only has one orbital shell. http://www.chem.arizona.edu/chemt/Flash/photoelectron.html Notice that there are 2 peaks shown for Li that correspond to the two orbital shells. 1s level 2s level 1s level As you read one of these diagrams remember to think about which energy level should take more energy to remove an electron. http://www.chem.arizona.edu/chemt/Flash/photoelectron.html Notice how large the energy value is for removal of the second electron (on both spectra) compared to removal of the first electron. (Remember that the 2nd e- is from an inner shell. X axis scale change http://www.chem.arizona.edu/chemt/Flash/photoelectron.html Just a Note • The PES spectra for B, C, N, O, F and Ne contain a second peak, of gradually increasing energy because of the increasing nuclear charge, that has the same intensity as the peak for the n=1 shell. • In each case there is a third peak (of gradually increasing energy) that corresponds to the electrons that are the easiest to remove from the atoms. Spencer, p.91 http://www.chem.arizona.edu/chemt/Flash/photoelectron.html It’s Interactive. Give it a try. Learn More About Spectroscopy • http://www.chem.qmul.ac.uk/surfaces/scc/sc at5_3.htm • http://www.rsc.org/learnchemistry/collections/spectroscopy/introducti on# More Info • The following website offers more background information about PES • http://www.chem.qmul.ac.uk/surfaces/scc/sc at5_3.htm Additional Info • I found a guided inquiry activity about Photoelectron Spectroscopy in Moog's Chemistry; a Guided Inquiry; 4th edition. • http://www.gobookee.net/chemistry-aguided-inquiry-4th-edition-solutions/ Resources • Spencer, J. N., Bodner, G. M., Rickard, L.H., (2010). Chemistry: Structure and dynamics. (5th ed.). Wiley. • University of Arizona Department of Chemistry and Biochemistry. (2013) Photoelectron Spectra. Retrieved from http://www.chem.arizona.edu/chemt/Flash/photoelec tron.html • http://bit.ly/1195Ahl • http://www.chem.qmul.ac.uk/surfaces/scc/scat5_3.ht m