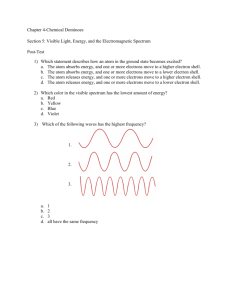
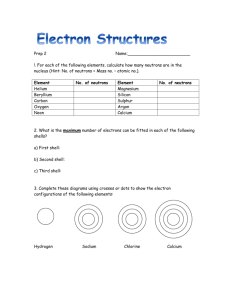
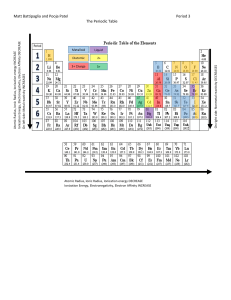
Document
advertisement

http://www.google.com/search?q=ebola+zombie+apocalypse&safe=active&es_sm=93&source=lnms&tbm=isch&sa=X&ei=EA5FVNS2KZCWigKqtIDgBA&ved=0CAoQ_AUoAw&biw=1280&bih=923&surl=1#facrc=_&imgdii=_&imgrc=oKxA00SOC1uuqM%253A%3BdqNktoZjsVokLM%3Bhttp%253A%252F%252Fcdn.meme.am%252Finstances%252F53266243.jpg%3Bhttp%253A%252F% 252Fmemegenerator.net%252Finstance%252F53266243%3B400%3B400 Log into Skyward This is not a punishment!!!! I need to make sure that this is academically viable in order for you to be able to keep this reward going. Just do your best Entry Task On a separate sheet of paper answer the following questions in complete sentences: 1. 2. How has the model of the atom changed over time? How is the Periodic Table Organized? Turn In Goal Sheets for History of the Atom Remember the goal of these sheets is to show how your knowledge progressed over the course of the unit If you wanted an “A” you needed to write your own personal goal and show the proof: Extra work to show your knowledge Conference with me about what you know Turn In Goal Sheets for History of the Atom There can be no blanks you must answer every questions Make sure you answer the questions (Learning Goals/Targets) to show you understand the material. That’s why I wir TURN THEM IN THURSDAY Update Your Table of Contents Still a part of the same unit Update Your Table of Contents 10/20/14 Bohr Model Add pg# the new Learning Target: How does the Bohr model relate to atomic structure and the organization of the Periodic table? Glossary ● atomic number – The number of protons in an atom. ● ● ● ● ● ● Sometimes called the proton number. electron arrangement – A shorthand way of writing the number of electrons in an atom’s electron shells. element – A substance made up of only one type of atom. group – A column in the periodic table containing elements with the same number of outer shell electrons and similar chemical properties. period – A row in the periodic table containing elements with the same number of full electron shells. periodic table – The table that lists all the elements in order of increasing atomic number, arranged into groups and periods. property – Any characteristic of an element. Bohred… again? 1. Atomic Number= Protons= electrons 2. Atomic Mass-Protons=Neutrons 3. First shell only holds 2 electrons 4. Next shells can hold 8 electrons Rules for Bohr Diagrams Know your atomic number 2. Know your atomic mass 3. First shell only holds 2 electrons 4. Next shells can hold 8 electrons 1. Hydrogen http://upload.wikimedia.org/wikipedia/commons/4/40/Electron_shell_001_hydrogen.png http://upload.wikimedia.org/wikipedia/commons/4/40/Electron_shell_001_hydrogen.png Helium http://0.tqn.com/d/chemistry/1/7/w/2/1/helium-atom.jpg http://rlv.zcache.com/helium_individual_element_of_the_periodic_table_napkinr05d22bec74a24d9a8a23ac715411e2fe_2cf00_8byvr_512.jpg Lithium http://chemicalelements.com/elements/li.html http://upload.wikimedia.org/wikipedia/commons/thumb/b/b6/Lithium.svg /424px-Lithium.svg.png