Electronegativity and Bond Polarity

Electronegativity and Bond
Polarity
Bond Polarity
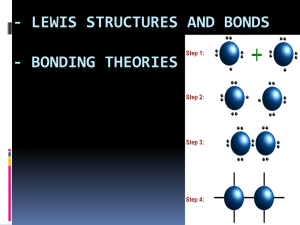
• So far we have assumed that when atoms share a pair of electrons they share the electrons equally.
• However, different atoms have different attraction for the electrons to be shared, and so do not share equally.
Electronegativity
• Electronegativity is a measure of the ability of an atom to attract the electrons in a bond.
• What are the trends that we see for electronegativity in the periodic table?
• Comparisons between electronegativity values can be used to make generalizations about the type of bonding and types of atoms forming a bond.
Electronegativity
• Elements such as fluorine, oxygen and nitrogen have high electronegativities, whereas metals have low electronegativities.
• For atoms in a molecule to share the bonding electrons equally, the electronegativities must be identical.
▫ This description is true for the diatomic molecules of an element such as N
2
, O
2
, Cl
2
, H
2 and so on.
Electronegativity
• When the electronegativities are similar, the sharing of bonding electrons is approximately equal.
▫ Such as carbon and hydrogen
• The greater the difference in the electronegativities of the atoms in a compound, the more uneven will be the sharing of electrons between them.
Electronegativity
• The extreme of unequal sharing is the formation of ions.
• When ions are formed, one ion loses its valence electrons completely and the other gains valence electrons.
• When the difference in electronegativities is great (approximately 1.8 and above), the compound is most likely to be ionic.
Electronegativity
• Similarly, the closer the electronegativity values of the two atoms the more likely they are to form a covalent compound by sharing electrons.
• The bonds formed between electronegativity differences of between 0.5 and 1.8 are more likely to be polar covalent, while those with an electronegativity difference of zero will form pure covalent bonds.
Electronegativity
• Most bonds are somewhere along a bonding type continuum.
• For this reason electronegativity values are only used as a general guide for identifying bonding type.
Electronegativities of Selected
Elements
Sample Problem
• Use electronegativity values and the location of the element in the periodic table to identify the compound made by each pair of elements as either covalent or ionic.
▫ Sodium and sulfur
▫ Sulfur and oxygen
▫ Lead and oxygen
Sample Problem
Element
Pair
Sodium and sulfur
Sulfur and oxygen
Lead and oxygen
EN
0.9
2.5
2.5
3.5
1.8
3.5
Difference in EN
1.6
Classification Bonding
Ionic
1.0
1.7
Metal
Non-metal
Non-metal
Non-metal
Metal
Non-metal
Covalent
Ionic
Bond Polarity
• If the electrons are shared unevenly in a covalent bond, the bond is said to be a polar covalent bond, or a permanent dipole.
• Such a bond can be identified using the symbol δ
(delta).
• In particular δ- and δ+ are used to indicate a slight negative and a slight positive charge respectively.
Bond Polarity
• If a polar covalent bond occurs in a diatomic molecule, one part of the molecule will be
more negative than the other, due to having a larger share of the bonding electrons.
▫ This is the case with molecules such as HCl and
HBr.
• The molecule is then described as a polar
molecule.
Bond Polarity
• When there is more than one polar covalent bond in a molecule, the shape of the molecule must be considered.
• It is possible to have molecules that contain polar bonds but overall are nonpolar—the permanent dipoles cancel each
other out.
Bond Polarity
• An example of such a molecule is methane.
• This molecule contains four polar C-H bonds, with carbon being slightly more electronegative than hydrogen.
• In three dimensions, the tetrahedral arrangement of the bonds in this molecule means that the slight positive charges of the hydrogen atoms are perfectly balanced out by each other and are cancelled out by the
partial negative charge on the carbon atom.
Bond Polarity
• In fact, any molecule that is perfectly
symmetrical will be non-polar overall.
Methane
Bond Polarity
• Chloromethane (CH
3
Cl) is a similar molecule to methane, but it is polar.
• Note that this molecule is not symmetrical in three dimensions and so the dipoles cannot cancel each other out.
• Chlorine (3.0) is more electronegative than either carbon (2.5) or hydrogen (2.1) and so draws the bonding electrons towards it.
Chloromethane
Carbon dioxide
• Another important example of a non-polar molecule that nevertheless contains polar bonds is carbon dioxide.
Practice Problems
• Identify which atom in each of the following bond pairs will carry a slight negative charge and which a slight positive charge.
a) C-H b) B-O c) P-Cl d) S-H e) Si-O
Answers a) C slight negative charge, H slight positive charge b) O slight negative charge, B slight positive charge c) Cl slight negative charge, P slight positive charge d) S slight negative charge, H slight positive charge e) O slight negative charge, Si slight positive charge
Practice Problems
Molecule
Name
Structural
Formula
Are bonds polar?
Is the molecule symmetrical?
Is the molecular polar overall?
Oxygen (O
2
)
Dibromomethane
(CH
2
Br
2
)
Carbon disulfide
(CS
2
)
Ammonia
(NH
3
)
Answer