Lewis Dot Structures
advertisement
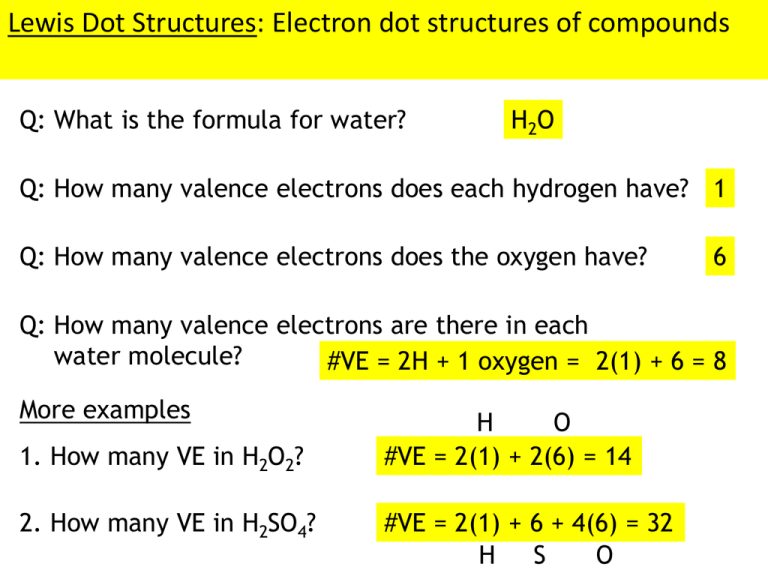
Lewis Dot Structures: Electron dot structures of compounds Q: What is the formula for water? H2 O Q: How many valence electrons does each hydrogen have? 1 Q: How many valence electrons does the oxygen have? 6 Q: How many valence electrons are there in each water molecule? #VE = 2H + 1 oxygen = 2(1) + 6 = 8 More examples 1. How many VE in H2O2? 2. How many VE in H2SO4? H O #VE = 2(1) + 2(6) = 14 #VE = 2(1) + 6 + 4(6) = 32 H S O The ‘Keep ‘em Happy’ approach to Lewis structures: 1. Add up the number of valence electrons present 2. Draw the stick-skeleton of the molecule 3. Satisfy the octet rule for all atoms in the molecule Exceptions: H only needs 2e- and B only needs 6e4. Count up the number of electrons present in the Lewis structure - If there aren’t enough e- (the molecule is ‘unhappy’), add the missing electrons to the central atom - If there are too many e- (the molecule is ‘too happy’), take the excess away from the central atom and then form double bonds with the terminal atoms to satisfy the octet. Examples: SF4 #VE = 34 Note: 1 stick = 2 electrons in a bond F S F F F 32 34 SO2 #VE = 18 O 20 S O 18 This molecule isn’t ‘happy’ because it doesn’t have enough electrons. This is the only possible structure because Fluorine NEVER forms double bonds This molecule is too happy, take away an electron pair from the central atom… …but now sulfur is unhappy with only 6 eMake sulfur happy by using one of the pairs on oxygen to form a double bond. Recall that the shape of a molecule can play a very important role in determining its properties. Example: The odor of the compounds outlined below depend upon their 3D shape Molecules will adopt whatever shape allows them to minimize the repulsion between electrons in adjacent bonds. H H C H H Ex: CH4 109.5° 90° You might think this is the farthest that the hydrogens can get away from each other But if you think in 3-dimensions, this shape actually causes less repulsion between the bonding pairs of electrons. A useful model for predicting the shape of molecules is the… •Molecules will adopt a shape that is lowest in energy •A low energy shape is one that minimizes the valence shell electron pair repulsion (VSEPR) between adjacent atoms (electrons in bonds and in lone pairs repel each other). The 5 Main Shapes Linear 180° Trigonal planar Tetrahedral 120° 109.5° Octahedral 90°, 180° Trigonal bipyramidal 120°, 180° Molecules adopt a geometry that minimizes electron-electron repulsions this occurs when e- pairs are as far apart as possible. Steps to determining molecular geometry: 1. Draw a Lewis structure 2. Determine the “AXE” notation •A = central atom •X = # atoms bonded to the central atom •E = # of lone pairs on central atom 3. Determine the geometry using the AXE chart Examples: PH3 X H E P A X H AX3E Trigonal pyramidal H AX2E2 bent HX H2 S H S Let’s look at a few examples… AX3 AX2E Trigonal planar bent H O H AX3E AX2E2 Trigonal pyramidal bent Going from AXE notation to hybridization of central atom: 1. Add up X and E subscripts on AXE notation Ex: AX5E1 5+1=6 you have to have 6 orbitals to hold 6 “things” 2. Combine orbitals until the superscripts add up to the same amount (start with s, then p, then d; Maximum of s1, p3, d5) Ex: s1 p3 d2 1+3+2=6 You have 6 hybrid orbitals A few more examples: AX3E s1p3 AX2E3 s1p3d1 AX3 s1p2 AXE s1p1 The Name Game: Covalent Molecules 1. The first element in the formula is also first in the name and retains the name of the element. 2. The ending of the second element is changed to –ide. 3. Prefixes are used to indicate the number of each element in the molecule. Mono- 1 Hexa- 6 Di- 2 Hepta- 7 Tri- 3 Octa- 8 Tetra- 4 Nona- 9 Penta- 5 Deca- 10 Working out the kinks with a few covalent compounds: 1. CO2 Carbon dioxide 2. N2O4 Dinitrogen tetroxide 3. SF6 Sulfur hexafluoride 4. PCl5 Phosphorus pentachloride 5. SO3 Sulfur trioxide 6. H2S Dihydrogen monosulfide 7. BCl3 Boron trichloride 8. NO Nitrogen monoxide 9. CF4 Carbon tetrafluoride 10. XeF4 Xenon tetrafluoride Now…change it up a bit and write the formula from the name: 1. Sulfur dioxide SO2 2. Diphosphorus tetroxide P 2 O4 3. Nitrogen trichloride NCl3 4. Krypton tetrafluoride KrF4 5. Dinitrogen trioxide N 2 O3 6. Dihydrogen monoxide H2O 7. Boron tribromide BBr3 8. Carbon monoxide CO 9. Silicon tetraiodide SiI4 10. Disulfur tetroxide S2O4