Intermolecular Forces – Forces of attraction between molecules
advertisement
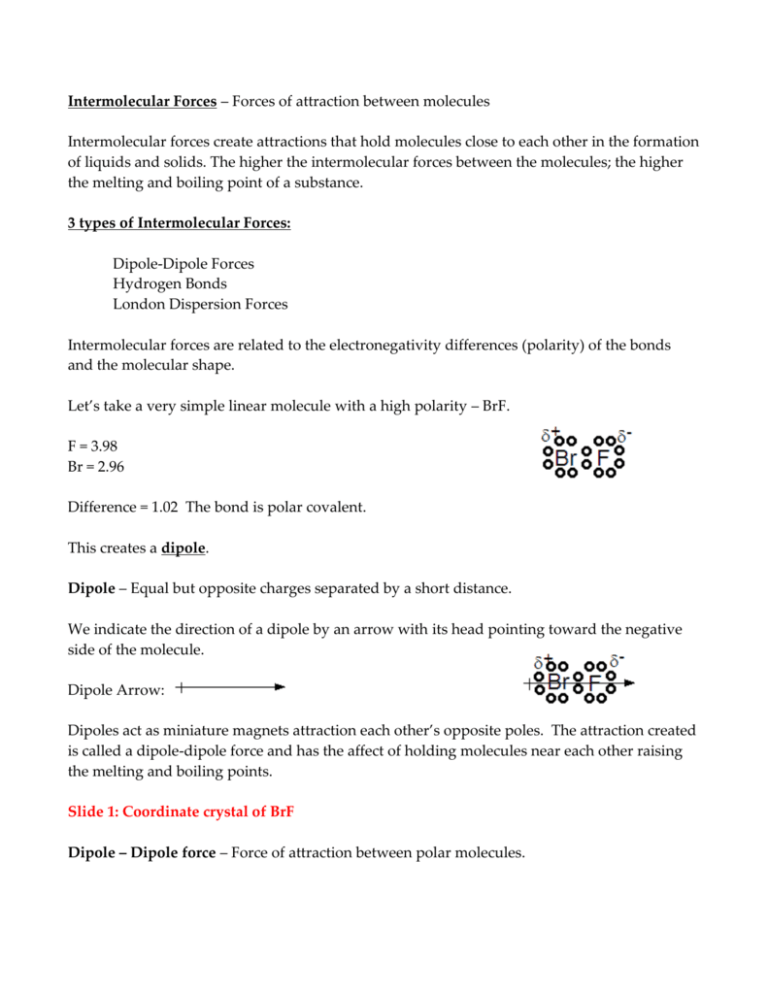
Intermolecular Forces – Forces of attraction between molecules Intermolecular forces create attractions that hold molecules close to each other in the formation of liquids and solids. The higher the intermolecular forces between the molecules; the higher the melting and boiling point of a substance. 3 types of Intermolecular Forces: Dipole-Dipole Forces Hydrogen Bonds London Dispersion Forces Intermolecular forces are related to the electronegativity differences (polarity) of the bonds and the molecular shape. Let’s take a very simple linear molecule with a high polarity – BrF. F = 3.98 Br = 2.96 Difference = 1.02 The bond is polar covalent. This creates a dipole. Dipole – Equal but opposite charges separated by a short distance. We indicate the direction of a dipole by an arrow with its head pointing toward the negative side of the molecule. Dipole Arrow: Dipoles act as miniature magnets attraction each other’s opposite poles. The attraction created is called a dipole-dipole force and has the affect of holding molecules near each other raising the melting and boiling points. Slide 1: Coordinate crystal of BrF Dipole – Dipole force – Force of attraction between polar molecules. Hydrogen is special due to its small size and the fact that it is filled with only 2 electrons in its first energy level. When hydrogen is combined in a polar molecule its small size allows its nucleus to get very close to the unshared electron pair on an adjacent molecule forming a very strong dipole-dipole attraction, called a Hydrogen bond. Hydrogen Bond – A strong dipole-dipole force between Hydrogen and highly electronegative atom to which it is not directly bonded. Slides 2 – 11 Nonpolar molecules will show some minimal attractions due to London Dispersion forces. London dispersion forces- Short term attractions between molecules due to short lived (momentary), unequal distribution of electrons. As electrons randomly orbit about the molecule they are not always equally distributed. When more electrons end up on one side of a bond then the other side it creates a short lived polarity. These short lived polarities create weak attractions between adjacent particles. Metallic Bond – A bond between the atoms of a metal in which metals freely share their valence electrons. Slides 12 and 13 Metals have a small number of very weakly held valence electrons with vacant p and d orbitals. These vacant orbitals overlap with the vacant orbitals of adjacent atoms allowing them to easily exchange these electrons with each other creating a “sea of electrons” surrounding positive metallic ions. This gives the metal its high conductivities, luster and photo-electric effects. Metallic Bond – attraction between metal atoms and the surrounding “sea of electrons”. Order of Bond Strength (from high to low) Covalent Bond – strong bond within the molecule holding the molecule together. Ionic Bond – Strong attractions spread throughout a crystal. Metallic Bond Hydrogen Bond Dipole-Dipole force London dispersion force









