Bonding
advertisement

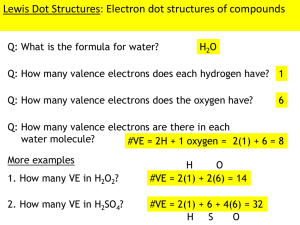
Bonding By John Patrick Fahy III of Galway Coulomb’s Law • • • • • Attractive force is proportional to (+q)(-q)/r^2 +q = magnitude of the positive charge -q = magnitude of the negative charge r = distance between the charges All bonds occur because of electrostatic attractions, electrostatic forces are governed by this law. What does this mean? • Bigger charges mean stronger bonds, smaller charges mean weaker bonds • Charges close together means stronger bonds, charges far apart mean weaker bonds Bonds Within Molecule • Atoms join to form molecule because atoms like to have a full outer shell of electrons. This usually means having eight electrons in the outer shell. Atoms with too many or too few electrons either give up, gain, or share electrons with other atoms. Types of Bonding Ionic bonds • Occur between atoms with very different electronegativities; this means usually metals and nonmetals • one atom gives up electrons and becomes a cation while the other the accepts electrons and becomes an anion Covalent Bonds • Occurs when 2 atoms share electrons • Each atom counts the shared electrons as part of their valence shell Drawing Lewis Structures • 1. Count the valence electrons in the molecule • 2. If it is an ion either subtract or add electrons depending on its charge • 3. Draw the skeletal structure of the molecule and place one single bond between each pair of bonded atoms, the least electronegative atom usually serves as the central atom • 4. Add electrons to the surrounding atoms until each has a complete outer shell • 5. Add the remaining electrons to the central atom • 6. Look at the atom • (a) if the central atom has less than eight electrons remove an electron pair from an outer atom and add another bond between that and the central atom. Do this until the central atom has a complete octet • (b) if the central atom has a complete octet, you’re finished • (c) if the central atom has more than 8, that’s okay too Molecular geometry • When atoms come together to form a molecule, the molecule will assume the shape that keeps its different electron pairs as far apart as possible, the model based on this idea to predict molecular geometries is known as the valence shell electron-pair repulsion theory (VSEPR) Geometry • If the central atom has 2 electron pairs than its hybridization is sp and its shape is linear • Ex. BeCl2, CO2 • If the central atom has three electron pairs then it has sp2 hybridization and its shape is trigonal planar, if one of the pair is a lone pair then the shape is categorized as bent • Ex. BF3, SO3, SO2 • If the central atom has 4 electron pairs then it has sp3 hybridization and its shape is tetrahedral, if one of the pairs is a lone pair then its shape is trigonal planar, if 2 of the pairs are lone pairs then it shape is bent • Ex. CH4, NH3, P • Cl3, AsH3, H2O, OF2 • If the central atom has 5 electron pairs then it has dsp3 hybridization and it is trigonal bipyramidal, 1 lone pair = seesaw, 2 lone pairs = T-Shaped, 3 lone pairs = linear • Ex. PCl5, PF5, SF4, ClF3, ICl3, XeF2 • If the central atom has 6 electron pairs then it has d2sp3 hybridization and is octahedral • 1 lone pair = square pyramidal • 2 lone pairs = square planar • Ex. SF6, BrF5, IF5, XeF4 Intermolecular Forces Dipole-Dipole Forces • Occur between neutral polar molecules • The positive end of one molecule is attracted to the negative end of another molecule • Molecules with greater polarity have greater dipoledipole attractions London Dispersion Forces • Occur between neutral, non-polar molecules • Very weak, occur because of the random motions of electrons on atoms within molecules