Experiment #6 - Molecular Geometry

Experiment #6 - Molecular Geometry
Introduction:
Note: Most of the technical information about Lewis structures, geometry, formal charge and hybridization can be found in any textbook about general chemistry. The information presented here is just a "different" way of looking at molecular geometry.
Drawing Lewis structures:
It is very important to be able to draw Lewis structures. A properly drawn Lewis structure will also greatly assist in getting the correct molecular geometry, hybridization,
VSEPR formula, and also help in drawing the 3-dimensional structure.
Steps to drawing the Lewis structure:
1. Select the central atom. The central atom is usually the least electronegative (the least electronegative atoms are found towards the left and "down" in the periodic table. Also, the central atom can NEVER be hydrogen (H) or fluorine (F).
2. Connect the "outside" atoms to the central atom by using single (2 e
) bonds. Instead of a straight line, 2 dots can also be used.
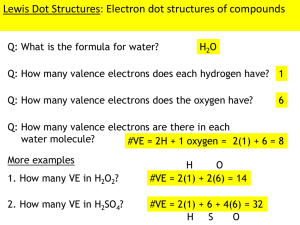
3. Count up the number of valence electrons for each of the individual atoms, then add them up.
4. Now you are ready to distribute the electrons. Place electrons (represented by dots) until each of the "outside" atoms have 8 electrons around them (the exception is if the outside atoms were hydrogen, then each hydrogen atom only needs 2 e
around them).
The bonded electrons DO COUNT when determining how many electrons are around outside atoms.
5. Now we count how many electrons are in our Lewis structure. Compare the number to the number of valence electrons counted in step 3.
6. If there are valence electrons left over, the extra electrons are put around the central atom as unbonded electrons. At this point you need to make sure that all atoms have 8 electrons around them (except for hydrogen, which can have 2 electrons around it). If the central atom has more than 8 electrons around it, but the central atom is in the 3rd period
(Al, Si, P, S, Cl) or lower, than that is fine.
7. If the central atom has less than 8 electrons around it, AND the outside atom is NOT
H, F, Cl, Br, or I, then you need to bring unbonded electrons from the outside atoms and make a double bond between that outside atom and the central atom. This does two
things. First, it ensures that the outside atom still has 8 electrons around it and also gives the central atom 2 more electrons around it.
Experiment #6 - Molecular Geometry (cont.)
8. Next, you may have to "adjust" the structure like in step #7 above to ensure that the formal charges on each of the atoms are as close to zero as possible. Ideally, all atoms in a neutral molecule will have zero charge.
Procedure:
From the list below, your laboratory instructor will assign a number of molecules for you to draw. For each of the molecules, fill out the information as noted in the report sheet
Here is the list of molecules:
1. BeF
2
2. BF
3
3. SO
2
4. SO
3
5. SO
4
2
6. NO
7. NO
8. CO
3
2
3
2
9. CS
2
10. SiH
4
11. SnCl
2
12. CH
3
13. PCl
4
+
14. H
3
O
+
15. O
3
16. NO
2
+
Note: The "AXE" notation is defined as
17. PON
18. AlCl
3
19. PO
3
3
20. PN
2
21. ClO
3
22. H
2
O
23. SnF
24. AlO
4
2
25. HCN
26. PF
3
27. CH
28. CF
29. BO
4
30. NH
4
+
31. GeF
2
2
4
Cl
32. CH
3
+
2
A = The central atom
X = Number of atoms directly attached to the central atom
E = Number of lone electron pairs on the central atom
For example: AX
2
E means there is one lone electron pair on the central atom and 2 (two) atoms directly attached to the central atom. It does not matter whether they are attached using single, double, or triple bonds.
Table of Molecular Geometries
AXE Notation Geometry Bond Angle Polarity *
AX
2
AX
3
AX
2
E
AX
4
Linear
Trigonal planar
V-shaped
Tetrahedral
180
120
~ 120
109.5
Non polar
Non polar
Polar
Non polar
AX
3
E
AX
2
E
2
Trigonal pyramidal
Bent
~ 109.5
~ 109.5
Polar
Polar
REPORT
Experiment #6 - Molecular Geometry (cont.)
Name______________________________ Partner____________________________
Molecule
Assigned
Lewis Structure
AXE
Geometry
Polarity
Molecule
Assigned
AXE
Geometry
Polarity
Molecule
Assigned
AXE
Geometry
Polarity
Lewis Structure
Lewis Structure
Experiment #6 - Molecular Geometry (cont.)
Molecule
Assigned
AXE
Lewis Structure
Geometry
Polarity
Molecule
Assigned
AXE
Geometry
Polarity
Molecule
Assigned
AXE
Geometry
Polarity
Lewis Structure
Lewis Structure
Experiment #6 - Molecular Geometry (cont.)
Molecule
Assigned
AXE
Lewis Structure
Geometry
Polarity
Molecule
Assigned
AXE
Geometry
Polarity
Molecule
Assigned
AXE
Geometry
Polarity
Lewis Structure
Lewis Structure
Experiment #6 - Molecular Geometry (cont.)
Molecule
Assigned
AXE
Lewis Structure
Geometry
Polarity
Molecule
Assigned
AXE
Geometry
Polarity
Molecule
Assigned
AXE
Geometry
Polarity
Lewis Structure
Lewis Structure