Lewis Structures
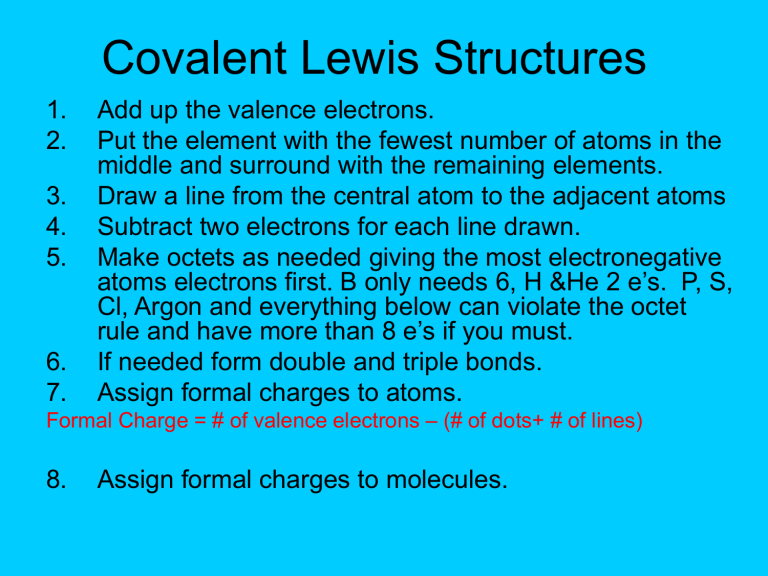
Covalent Lewis Structures
1.
Add up the valence electrons.
2.
Put the element with the fewest number of atoms in the middle and surround with the remaining elements.
3.
Draw a line from the central atom to the adjacent atoms
4.
Subtract two electrons for each line drawn.
5.
Make octets as needed giving the most electronegative atoms electrons first. B only needs 6, H &He 2 e’s. P, S,
Cl, Argon and everything below can violate the octet rule and have more than 8 e’s if you must.
6.
If needed form double and triple bonds.
7.
Assign formal charges to atoms.
Formal Charge = # of valence electrons – (# of dots+ # of lines)
8.
Assign formal charges to molecules.
VSEPR
1. Beryllium chloride
2. Aluminum chloride
3. Methane (CH
4
)
4. Phosphine (PH
3
)
5. Water
6. Niobium (V) Bromide
7. Sulfur hexafluoride
Notes
Water
CO
2
CO
H
2
O
SF
6
BeCl
2
CH
4
AlCl
3
PH
3
NbBr
5
C
2
H
3
O
2
-1
CO
3
-2
ClO
2
-1
CN -1
HNO
3
PO
4
-3
Na
2
SO
3
(NH
4
)
2
O
2
SeF
6
I
2
S
BF
3
PI
5
CCl
4
NCl
3
SiO
2
BeCl
2
(Ionic) AlCl
3
(Ionic)
CH
4
(Covalent) PH
3
(Covalent)
H
2
O (Covalent) NbBr
5
(Ionic)
SF
6
(Covalent)
HCl
PI
3
O
3
N
2
Rb
2
S
SiCl
4
C
2
H
2
O
2
I
2
O
BI
3
SbCl
5
TeCl
6
TeO
4
-2
SeS
2
NO
3
-1
AsO
4
-3
H
2
S
BBr
3
PCl
5
TeF
6
SiI
4
SiO
NCl
3
CaO
TlAt
3
PbCl
4
Rb
2
O
RaBr
2
SrCl
2
SbI
5
NaF
Al
2
O
3
Add up the total number of valence electrons available (EA). Be sure to take into account the total number of atoms present.
Exception: if the compound is negatively charged, add 1 electron for each negative value
Exception: if the compound is positively charged, remove 1 electron for each negative value
Example: CH4 C= 4 VE H= 1VE = 4 + 4(1) = 8 EA
Example: CO2
Example: SO42-
Draw out a skeletal arrangement of lewis structure. Place the least electronegative atom in the center, surrounded by the remaining atoms. Draw lines to represent the bonds between the central atom and each surrounding atom.
Exception: Hydrogen can never be a central atom because it is found in the first energy level and can only have 2 electrons at most.
Draw the skeletal arrangement in the following examples
CO2
H2SO4 (Just underline the central atom)
NCl3
PF5
Count and draw in the number of electrons necessary (EN) for each to have a full octet.
These are the electrons needed. Be sure to include the bonded electrons.
Example: CO2
Subtract the number of valence electrons available (from step one) from the number of electrons needed (EN –EA). This is the number of valence electrons that you still need. We make up for these by adding double or triple bonds. If you get a negative number, you have extra electrons. These may become part of your expanded octet.
Example: CO2
To add double or triple bonds: divide the number you get from step 4 by 2 (why? Because we are adding more bonds and each bond represents 2 electrons). The number you get is