Ch 2 The Chemical Context of Life
advertisement

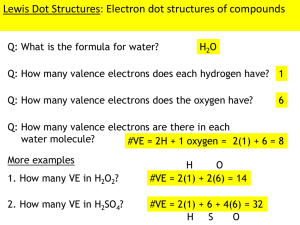
Ch 2 The Chemical Context of Life I. Overview All living organisms are subject to the laws of chemistry & physics. B. A basic knowledge of both helps us to better understand how living things work. 1. What class of levers do you find most often in the human body? 2. Why use those? 3. How do moth species recognize mates of the same species? 4. Read the test case on the “Devil’s gardens” in the rain forest in Ch 2. A. Section 2.1 The nature of Matter A. Matter- anything that has mass and takes B. up space 1. Matter consists of chemical elements in pure form or in combinations called compounds 2. All organisms have mass & take up space, therefore all organisms are made of matter Matter is made of elements. 1. Elements are substances that can’t be broken down into other substances by chemical reactions C. C. D. A compound is a substance consisting of two or more different elements chemically combined in a fixed ratio. 1. Compounds will have chemical and physical characteristics different from those of their elements. 2. ex. Salt is made of Sodium (Na) and Chlorine (Cl). Sodium explodes in water, chlorine is a poison. Salt is a harmless, edible compound. The smallest unit of an element is an atom. The smallest unit of a compound is a molecule. Making Salt II. Essential Elements of Life A. B. 25 of the 92 elements are essential to life. Carbon, hydrogen, oxygen and nitrogen make up 96% of all living matter. (CHON) or (CHONS) C. Calcium, potassium, phosphorous, and sulfur makes up most of the remaining 4%. (CaPSK) D. Trace elements make up about 0.05%. Insufficient nitrogen in plants Iodine deficiency in people What happens when essential elements are missing? III. A. B. An element’s properties depend on the structure of its atoms. An atom is the smallest unit of matter that retains the properties of an element. Atoms are made of subatomic particles which include: 1. Neutrons: 0 charge, found in the nucleus 2. Protons: + charge, found in the nucleus 3. Electrons: - charge, found in the cloud around the nucleus C. Atomic number- the number of protons in an element’s nucleus- the number is unique for each element D. E. F. All atoms of an element have the same number of protons, but may have different numbers of neutrons in the nucleus. Isotopes- when two atoms of an element have different numbers of neutrons Radioactive isotopes- may decay spontaneously giving off particles & energy. These isotopes are used for 1. dating fossils 2. diagnosing medical problems 3. tracing atoms through metabolic processes Highlighted area represents cancerous throat tissue. IV. Energy Levels of Electrons A. B. Energy-an ability to do work or cause change Potential energy- energy due to the position or structure of matter 1. Electrons’ potential energy is due to their energy level or position in an electron shell. 2. Electrons losing energy, fall to a lower shell. C. D. The chemical behavior of an atom is due to the distribution of its electrons in the electron shells, especially the valence electrons An atom’s bonding capacity is called its valence. E. F. Valence electrons are those in the outermost or valence shell of the atom. Elements with a full valence shell are chemically inert. V. Electron Orbitals A. An orbital is a three dimensional space where electrons are found 90% of the time. B. Each electron shell has a specific number of orbitals Section 2.3 The formation and function of molecules depend on chemical bonding between atoms A. B. C. D. Atoms with incomplete outer shells (8 electrons) give, take, or share electrons Such interactions form chemical bonds. Chemical bonds- attractive forces holding atoms close together, making molecules Molecules consist of two or more atoms held together by chemical bonds. II. Covalent Bonds A. B. C. Covalent bond- the sharing of a pair of valence electrons by two atoms 1. The shared electrons each count as part of each other’s valence shell Types of covalent bonds 1. Single covalent bond- made by the sharing of one pair of electrons 2. Double covalent bond- made by the sharing of two pairs of electrons 3. Triple covalent bond- made by the sharing of three pairs of electrons Covalent bonds can form between atoms of the same or atoms of different elements III. Showing chemical bonds A. B. Structural formula- a notation used to represent atoms & bonding in a molecule ex. H H Molecular formula- an abbreviated formula for a compound ex. H2 IV. Polarity A. B. C. D. Electronegativity- how strongly an atom attracts electrons; it depends on the element’s position in the Periodic Table; the greater the electronegativity, the stronger the pull on the electrons Nonpolar covalent bond- electrons are shared equally between the two atoms Polar covalent bond- one atom is more electronegative than the other; the electrons are unequally shared Polar compounds- compounds that have polar covalent bonds. Due to unequal electron sharing, one atom has a slight negative charge, the other has a slight positive charge E. Water- a polar molecule 1. -Indicates a partially positive charge on the atom 2. -Indicates a partially negative charge on the atom 3.So, the oxygen atom is slightly negative (it has the electrons most of the time) and the hydrogen atoms are partially positive. V. Ionic bonds A. B. C. D. E. F. When there is a large difference in the electronegativity of the two atoms, one may take the valence electron(s) from the other. After the transfer of electrons, the two atoms have charges Ion- a charged atom or molecule Cation- a positively charged ion (J) Anion- a negatively charged ion (L) Ionic bond- a chemical bond formed by the attraction between an anion and a cation. (Breaks easily to form ions) IONIC BONDS ILLUSTRATION The red electron on sodium is transferred to Chlorine. This leaves 8 electrons in the remaining outer electron shell for sodium and completes the valence shell for chlorine with the 8th electron, making an ionic bond. VI. Weak bonds A. B. C. Chemical bonds vary in strength. Going from strongest to weakest, the order is covalentionichydrogenVanderwaals Covalent bonds form most of a cells bonds. Weak bonds are used to 1. reinforce the shapes of large molecules 2. help molecules adhere to each other B. C. Hydrogen bonds- form when the hydrogens of one polar molecule are attracted to strongly electronegative atoms of a nearby polar molecule. In cells, nitrogen and oxygen are electronegative Hydrogen bonds between polar molecules D. E. F. Unequally shared electrons in a molecule or atom cause “hot spots” of positive or negative charge Vanderwaals interactions- are attractions between molecules that are close together as a result of these “hot spots”. This is what gives us the strong attracti ve forces between the ridges on a geko’s toe pads & a wall. VII. Molecular shape & function The function of a molecule depends on its shape. Examples include DNA & proteins. B. The shape of a molecule depends on the positions of the valence electron orbitals for each of the atoms in the molecule. C. In covalent molecules, the s & p orbitals may affect each other (hybridize) to make specific shapes. A. Molecular Shapes Biological molecules recognize & react with each other based on the specific shape of the molecules. E. Molecules with similar shapes can have similar biological effects (see picture to the left) D. VIII. Section 2.4 Chemical Reactions A. B. C. D. E. F. Chemical reactions- making & breaking chemical bonds to make new molecules Reactants- starting molecules of a reaction Products- final molecules of a reaction Some chemical reactions go to completion & all reactants are converted to products Some reactions are reversible; the products of the forward reaction become the reactants of the reverse reaction. Chemical equilibrium- is when the forward & reverse reactions occur at equal rates. G. H. G. Some reactions are not reversible but may be paired with an opposite reaction. An example is photosynthesis & respiration. Photosynthesis- sunlight powers the conversion of carbon dioxide and water into glucose and oxygen. CO2 + H2O C6H12O6 + O2 Respiration- the breakdown of glucose using oxygen into carbon dioxide, water, and usable energy C6H12O6 + O2 CO2 + H2O Acknowledgements: This presentation is drawn almost entirely from the materials provided by Reese Campbell 8 th ed. DVD materials & notes